What are the body's 3 main acid-alkaline buffering systems?
3 main buffer systems
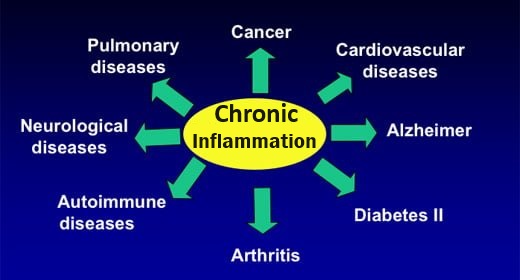
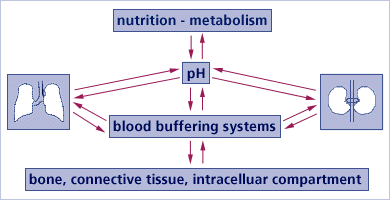
In functional equilibrium with each other, there are three main buffer systems contributing to the regulation of the acid-base balance:
(1) Chemical Buffer Systems In blood, lymph, and intra/extracellular fluids;
(2) Respiratory Compensation (Gaseous exchange in the lungs). Breathing out CO2 deals with much of our acid excess.
(3) Renal Mechanisms (Excretory functions of the kidneys) The kidneys serve primarily to excrete protons created during the breakdown of different acids. This excretory system is needed because the typical diet tends to present more H+ ions (protons) than alkalizing substances that might neutralize them.
(1) Chemical buffer systems
First line of defense against acid or base additions to body fluids. All body fluids contain acid-alkaline buffers. These buffers are chemicals that readily combine with an acid (or base) that function to prevent drastic changes in the pH of a body fluid when a strong acid (or base) is added. There are seven types of chemical buffers maintaining healthy body pH levels. These buffers (located in ECF, intracellular fluid and bone) neutralize, bind or dilute strong acid solutions in:
- Blood, lymph and tissues
- Lungs
- Kidneys
The 3 major chemical buffers are carbonic acid-bicarbonate, phosphate and protein.
(a) Carbonic Acid – bicarbonate buffers
(in blood, lymph and tissues)
This is the most important system, since if this system is stable, then the other systems are stable
Interacts directly with respiration through the formation of carbon dioxide. Also, carbonic acid is created from hydrogen carbonate, which breaks down into water and carbon dioxide, which is breathed out. Blood pH regulation is carried out by different buffer systems consisting of a weak acid and its corresponding base in a particular ratio;
This system controls pH instantaneously
This system is a mixture of carbonic acid (H2CO3) and the bicarbonate ion (HCO3– ) present as Salt HCO3 (Salt can be sodium, potassium, or magnesium).
- When the H+ ions of a strong acid are added to this mixture, it immediately combines with the bicarbonate ion to form carbonic acid (a very weak acid). And then the pH is only slightly decreased.
- Example of Acid buffer:
HCl + NaHCO3 → NaCl + H2CO3
strong acid + weak base → salt + weak acid
The carbonic acid-bicarbonate buffer is the only significant buffer for keeping the extra-cellular bicarbonate to carbonic acid ratio at the required 20:1
Bicarbonate ions are generated in the RBCs or stomach’s parietal cells from CO2 and H2O
- RBCs. RBCs contain enzyme carbonic anhydrase which speeds up the reaction of CO2 and water by 5000 times.CO2 + H2O → H2CO3 + H+ + HCO3–. Hemoglobin absorbs the H+ ions and the bicarbonate ions enter blood stream (this removes 70% of CO2 from blood; 7% of CO2 is dissolved in plasma; remaining 23% of CO2 combines directly with hemoglobin and is exhaled through the lungs).
- Stomach’s Parietal Cells. Whilst producing gastric acid,the parietal cells of the stomach simultaneously generate bicarbonate ions from CO2 + H2O, which diffuse into the blood stream.
Bicarbonate ions are used to neutralize H+ ions in blood, lymph, tissue fluids, and kidneys
Bicarbonate neutralizes H+ to form CO2. CO2 is exhaled through the lungs via the respiratory buffering system. E.g. H2SO4 + 2NaHCO3 →Na2SO4 + 2H2CO3 →2CO2 + 2H2O + Na2SO4.
Bicarbonate is also active in the kidney buffering system – where it is either reabsorbed to lower blood acidity or excreted with bound acids to maintain a balanced pH.
In an example of an alkaline-buffering system the chemical equation looks like this:
NaOH + H2CO3 → H2O + NaHCO3
strong base + weak acid → water + weak base
When a strong base is added to the mixture, it combines with the carbonic acid to form water and its neutral bicarbonate salt.
(b) Phosphate and ammonia buffers
(Primarily for blood passing through kidneys and intracellular fluid)
Phosphates can also work weakly in the blood and lymph. These buffers are especially important in intracellular fluids, where their concentration far outweighs bicarbonate buffers.
Acid Buffering:
HCl + Na2HPO4 → NaCl + NaH2PO4
strong acid + weak base → salt + weak acid
Na2HPO4 is actually the “salt” in the following dissociation reaction:
H2PO4– <=> H+ + HPO4 -2
Alkaline Buffering:
NaOH + NaH2PO4nbsp;→nbsp;H2Onbsp;+ Na2HPO4
strong base + weak acid → water + weak base
Phosphate. Acid blood (H+ ions) passing through the kidneys is buffered with salts of sodium dihydrogen phosphate(Na2HPO4) , creating H2PO4–, a weak acid, which is then excreted in urine ( H2PO4–â†→H++ HPO4-2).In the process, sodium is exchanged for an H+ ion removed from the extracellular fluid (ECF), forming a bicarbonate ion, which is then releasedinto the ECF. The kidney thus reduces the degree of acidosis in the body fluids.
Ammonia (NH3). Fermentation of amino acids reacts with the H+ ions to form ammonium ions, which are excreted into the urine, again increasing the bicarbonate concentration in ECF. By removing specific amounts of H+ ions from the blood and secreting them into the filtrate, the kidney can keep the pH of the blood at a constant level of 7.365.
(c) Protein Buffers (15%)
(intracellular fluid, lymph, blood)
The most plentiful buffers inside cells and in plasma. Proteins have negative charges and serve as both acidic (H+) and alkaline buffers.
E.g. histidin, glutathione, methionine, cysteine, taurine, hemoglobin.Most action is to bind or neutralize acids inside cells.
(d) Electrolyte Buffers
(in blood, lymph, extracellular / intracellular fluids)
The “X CO3’s “. Alkaline minerals (“X”) work to bind acids, which are then removed through the urine. The “Big 3″alkaline minerals are sodium, calcium and potassium, which are recycled by the kidneys back into the blood and lymph by binding them to CO2 (provided by 70% of the output from the body’s cellular energy-producing fermentation processes). In chronic acidosis, alkaline minerals are drawn from the bones to maintain life-sustaining blood pH.
E.g. The strong sulphuric and phosphoric acids (from the oxidation of sulphur-containing amino acids (e.g. in meat, eggs) and the oxidation of phospholipids) can be neutralized by alkaline minerals and then excreted by the kidneys.
MINERALS | EXAMPLES | COMPOUNDS | |
ACID -ve charge | Chlorine (Cl–) Sulfur (S–) Phosphorus (P–) | Attracted to the H+ ion | Hydrochloric acid (HCl) Sulfuric acid (H2SO4) Phosphoric acid (H3PO4) |
ALKALINE +ve charge | Calcium (Ca++) Potassium (K+) Sodium (Na+) Magnesium (Mg++) | Attracted to the negatively charged hydroxyl ion (OH-) | E.g. CaCO3 + H2SO4 →CaSO4 + H2O + CO2 |
(e) Low density lipoproteins of fat buffers
(in the blood, lymph, and extracellular fluids)
LDL and fat (especially electron-rich polyunsatured fats) bind acids, which are then excreted via the urine – If elimination is compromised, these fat-bound acids are moved into the body cavities, hips, thighs, stomach, etc. i.e. obesity.
(f) Hormonal buffers
Two kidney hormones especially help the kidneys maintain alkalinity and reduce excess acidity:
- ADH (antidiruretic hormone). Regulates rate of water excretion or retention
- ALDOSTERONE. Regulates the level of sodium ions (Na+) and potassium ions (K+) in the blood.
(g) Water
(in the blood, lymph, intracellular and extracellular fluids)
Water helps to maintain alkalinity in the blood, lymph, intracellular and extracellular fluids – by diluting excess acidity.
(2) Respiratory Compensation (Gaseous exchange in the lungs) – Buffers CO2 in blood
CO2 is constantly being produced in tissues and then transported via the blood to be expelled by the lungs – This provides the body with the biggest pH-buffering job – to maintain equilibrium in the concentrations of carbon dioxide, carbonic acid and bicarbonate resulting from their continuous formation and elimination. This is not usually a problem since CO2 is easily eliminated via the lungs, and pH(H+ ion concentration) can be adjusted by altering the number of breaths / minute to increase or decrease exhalation of acidic CO2.
Hydrogen ion Concentration Controls Breathing Rate
- An extracellular (EC) acid pH (high H+ concentration) stimulates the respiratory center in the medulla of the brain to speed up breathing rate (removing CO2 and thus carbonic acid, and so increasing pH by removing H+ ions)
- An EC alkaline pH (low H+ concentration) stimulates the respiratory center to slow down breathing rate
Consider the following reaction:CO2 + H2O < — > H2CO3 < — > H+ + HCO3–
In the tissues where carbon dioxide is abundant, the reaction is shifted to the right:
CO2+ H2O→H2CO3→H+ +HCO3–
In the lungs where H+ ions are liberated from hemoglobin, the reaction is shifted to the left:
CO2+ H2Oâ†H2CO3â†H+ +HCO3–
Hemoglobin absorbs H+ ions – This protein respiratory pigment of the red blood cells is a VERY IMPORTANT BUFFER in RBCs, particularly in carbonic acid buffering.
If breathing decreases below normal:
→ Increases CO2 → increases H2CO3 → increases H+ ions → increases acidity.
E.g. If breathing stops for 1 minute, extracellular pH → 7.1 (from its normal 7.4)
(Alternatively, over-breathing raises extracellular pH to ~7.7 in 1 minute)
(3) Renal Mechanisms – Compensate for acid pH by excretion of H+ by producing acidic urine
Normal Renal Mechanisms vs. Renal Compensation
- Normal Renal Mechanism. Distal tubules of the kidneys secrete H+ ions directly into the filtrate so that urine is acidified and the H+ ions are lost from the body.This is a normal process that occurs at a normal rate.
- Renal Compensation. Occurs when other mechanisms for acid-base balance fail and the rate of kidney excretion of H+ ions increases above normal,which are then eliminated from the body.

The kidneys are the ultimate acid-base regulatory organs – Although the body’s buffer systems can resist pH changes, by tying up excess H+ ions,the constantly generated H+ must eventually be eliminated from the body.The lungs can eliminate only carbonic acid (by eliminating carbon dioxide), but only the kidneys can rid the body of metabolic acids (phosphoric, uric, and lactic acids, and ketones) and prevent metabolic acidosis.
(1) By eliminating H+ ions or retaining bicarbonate. The kidneys control H+ ion concentration in extra-cellular fluids by either –
(a) H+ ion secretion – Excess H+ ions (secreted by kidney’s nephron tubule cells) combine with urinary buffers to be excreted as acids (i.e. acidifies the urine) and ammonium.
or (b) Bicarbonate (HCO3-) ion reabsorption. Excess, secreted H+ ions and bicarbonate ions [filtered into the glomerular filtrate] combine in the kidney tubules to form carbon dioxide [which is exhaled out] and water [which is urinated out].
E.g.HCl + NaHCO3 ↔ NaCl + H2CO3 ↔ CO2 + H2O + NaCl
Or (2) By exchanging H+ ions for Na+ or K+ ions. Another compensatory method for acidosis is to exchange H+ ions for sodium or potassium ions in the kidney tubules, producing a sodium/potassium imbalance and possible hyperkalemia;
– Effective blood buffering by the kidneys produces a urine pH of 4.6 – 8;
Note: the kidney can correct states of excess but not states of deficiency – Correction of an acidosis due to a loss of or insufficient base (Na+ or K+ +HCO3– ), e.g. by diarrhea, requires the administration of Na or K salts from which Na+ or K+ +HCO3– can be formed.
Kidney Stones
Formed in overly-alkaline conditions: are composed of magnesium and calcium phosphates, carbonates and oxalates, which are insoluble in alkaline fluids. A diet that acidifies the urine is desireable (e.g. cranberry juice) to reduce these type of stones.
Formed in overly-acid conditions: are composed of uric acid and crystine. A therapeutic diet would alkalize the urine.