Acidosis is what happens when body fluids are more acidic than they should be?
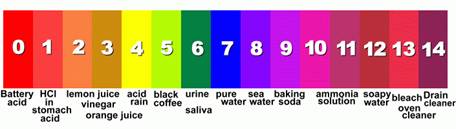
The acid-alkaline balance in body fluids is crucial to health
Body’s acid vs alkaline balance of prime importance to health
For optimal body function, the pH level of blood plasma and external and internal cellular fluids must be kept in the narrow pH range of 7.35 – 7.45 – acidosis occurs when this pH drops lower than 7.2 on a regular basis, and has detrimental consequences to health.
Symptoms that may indicate excess body acidity
|
|
Acidosis promotes detrimental changes in the body
Depletes bodys alkaline reserves
The ongoing buffering mechanisms use alkaline electrolyte minerals (including sodium, potassium, calcium and magnesium), bicarbonate, ammonia and proteins to correct an overly acidic pH due to strong acid presence.
Decreases cellular energy production
Oxygen levels decrease. Partial oxygen pressure (PO2) expresses amount of O2 that can react/combine with hemoglobin to form oxyhemoglobin, the form in which O2 is transported through body. This reaction rate decreases as blood plasma becomes more acidic, which decreases the amount of oxygen that can be delivered to cells, and thus decreases cellular energy production.
Cellular Sodium /Potassium Pumps are Compromised. Na+/K+ pumps in the cell membrane are central to cellular energy production of all cells.
- To function correctly these pumps must maintain the cell membrane potential, by keeping a low sodium / high potassium concentration of ions inside the cell. When hydrogen (H+) ions move into cells to compensate for an overly acidic blood plasma pH, they exchange places with potassium ions inside the cell (to maintain electrical equilibrium inside cell), and thus disturb Na/K pump function. Malfunctioning pumps leave us feeling tired and burning less fat and causes a sodium and calcium buildup within the plasma.
Acid build-up in tissues
Continual acid production can deplete the chemical buffers in the blood plasma, such that a healthy pH can no longer be maintained. To deal with this problem, excess acid is stored in the connective tissue and joints, including ligaments, tendons and muscles. As these acid stores build up, they can cause pain and inflammation in the storage areas.This may manifest as arthritis or fibromyalgia.
Overly acidic blood plasma is an arterial irritant
(may lead to atherosclerosis)
If blood plasma pH is consistently on the acidic end of the required 7.36 -7.42 range, then the blood plasma’s acidity acts as a chemical irritant, slowly “eating away”at the inner linings of the artery, vein and heart. This weakens their structures and sets the stage for the need to lay down arterial plaque to repair the damage.
Accelerates free radical damage
Acidosis causes partial lipid breakdown and destructive oxidative cascades, accelerating free radical damage of cell walls and intracellular membrane structures. Acidosis is a stepping stone towards premature aging, deterioration of eyesight and memory, creating skin wrinkles / age spots, dysfunctional hormonal systems, other age-related phenomena.
More mucus
Acid-producing foods create mucus, which congests and retards oxygen entry
More chloride ions
In normal pH, 99% of chloride ions are reabsorbed, less are reabsorbed in acidosis
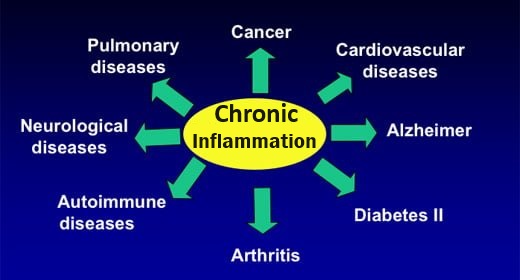
Health Problems that can result from mild acidosis | |
|---|---|
CARDIOVASCULAR DAMAGE |
|
RESPIRATORY PROBLEMS |
|
WEIGHT GAIN, OBESITY AND DIABETES |
|
CANCER |
|
BONE DISEASE |
|
KIDNEY STONES |
|
OTHER HEALTH PROBLEMS |
|