The many "Work Hats" of magnesium in the body

- Activates vitamin D
- Alkalizing
- Anti-inflammatory
- Prevents soft tissue calcification
- Calms nerves
- Powers cell “batteries”
- Cell membrane integrity
- Metabolism
- Detoxifies
- Antioxidant glutathione synthesis
- Heart health
- Hormonal balance
- Insulin
- Muscle relaxant
- Function of 300+ enzymes
- Regulates calcium
- Study-supported health benefits of magnesium
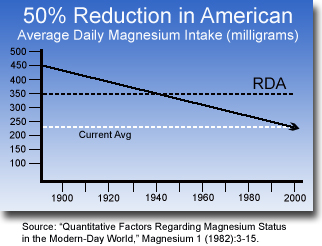
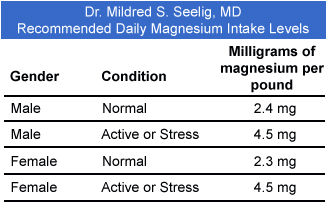
- Magnesium deficiency is common

Magnesium is an alkalizing mineral
Mg is a positively charged alkalizing mineral.
- Acid minerals have a negative electrical charge. (Attracted to the H+ ion). They include: chlorine (Cl-), sulfur (S-), phosphorus (P-), and form hydrochloric acid (HCl), sulfuric acid (H2SO4), and phosphoric acid (H3PO4).
- Alkaline minerals have a positive electrical charge (attracted to the negatively charged OH- ion). Nutritionally important alkaline minerals include calcium (Ca+), potassium (K+), magnesium (Mg+), and sodium (Na+). Na and Ca tend to be oversupplied in the typical western diet.
Mild acidosis disturbs Na+/ K+ pump function / cellular energy production. To compensate for an overly acidic blood pH, hydrogen (H+) ions are moved into cells, where they exchange places with potassium ions inside the cells (to maintain electrical equilibrium inside cell), thus disturbing Na+/ K+ pump function.

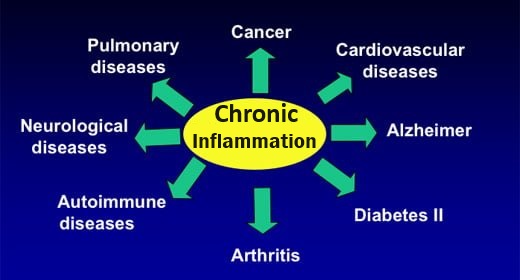
Magnesium is anti-inflammatory
Magnesium needed for inflammation-controlling localized “hormones”
Magnesium (also vitamin B6 and zinc) are required for the Δ6D enzyme. This enzyme converts essential fatty acids in foods into needed active forms, and which eventually convert to inflammation- controlling prostaglandins and leukotrienes (localized “Hormones”, called eicosonoids)
Eicosanoids – Cell’s “First Responders”
Magnesium intake / deficiency affects inflammatory marker CHP
Magnesium intake ↓ Chronic Inflammation marker CRP ↑
- According to the USDA both decreased magnesium intakes and blood magnesium levels have been associated with an increased CRP in people of all ages. Numerous studies have shown that a low magnesium status occurs often in people with diseases that have a chronic inflammation component, including heart disease, diabetes, high blood pressure, and osteoporosis.”
- According to publication by King et al, “Dietary magnesium and C- reactive protein levels”: “Most Americans consume magnesium at levels below the RDA. Individuals with intakes below the RDA are more likely to have elevated CRP, which may contribute to cardiovascular disease risk.” King et al, 2005
- Another study examined the association between serum magnesium levels and C- reactive protein (CRP) in non- diabetic, non- hypertensive obese subjects. Concluding: “The results of this study show that low serum magnesium levels are independently related to elevated CRP concentration, in non- diabetic, non- hypertensive obese subjects.” Relationship study
Magnesium intake ↑ Inflammation ↓
- Dietary magnesium connected to lowered diabetes risk. A study by Dr. Ka He of the University of North Carolina at Chapel Hill and colleagues found a connection between dietary magnesium and a lowered risk of diabetes/decreasing INSULIN resistance – also revealed that as magnesium intake increased, inflammation levels decreased. Dae Jung Kim, 2011
- Conclusion of another study with older, middle- aged American women: “Our results suggest that magnesium intake is inversely associated with systemic inflammation and the prevalence of the metabolic syndrome in middle- aged and older women.” Magnesium intake

Magnesium prevents calcification of soft tissues
A healthy cell has high magnesium and low calcium levels
- Up to 30% of cellular energy is used to pump calcium out of the cells. Inside the cells, normally, ATP energy produced by cell mitochondria is used to power the calcium pumps (Ca2+ATPase) that transport Ca++ ions across the plasma membrane to maintain an extracellular calcium concentration ~10,000 times greater than inside cells (Magnesium concentration inside cells ~10,000 times greater than the calcium concentration) – impaired cell membranes or low ATP production (due to lack of Mg for the enzymes required for ATP production or any other reason) means there is insufficient power to pump out the Ca++ ions and calcium accumulates inside the cell, preventing the cell from maintaining its normal calcium gradient. In this event, intracellular calcium increases (a benchmark at the time of death) and there is a relative deficiency of magnesium.If anyone is seriously ill, this deficit must be addressed with magnesium supplementation, noting that most oral forms are poorly absorbed.
High calcium / Low magnesium intake contributes to calcification of tissues
- Outside the cells, Mg keeps calcium dissolved. Calcium intake without sufficient magnesium encourages soft tissue calcification, since the higher the calcium level and the lower the magnesium level in the extra-cellular fluid, the harder is it for cells to pump the calcium out.
A relative deficiency of magnesium compared to calcium causes abnormal calcification in soft body tissues
- Extra-skeletal calcification. Beneficial calcification involves calcium and phosphorus and is a normal process for building healthy bones and teeth. As the ratio of Mg to Ca decreases inside cells (which happens as we age and with lower ATP energy production), calcium that is supposed to be deposited in your bones and teeth tends to accumulate in soft tissues (where there should not be calcification), and where it can cause many health issues, such as strokes, heart attacks and muscle spasms.
Health consequences of calcium accumulation inside cells (calcification)
Primarily caused by Mg deficiency or Mg/Ca imbalance. Unable to remove calcium from cells, abnormal calcium accumulations (calcifications) build up in soft tissues, and cell membranes become rigid. This affects cell transport systems, further decreasing Mg transport and other nutrients, with significant adverse effects in the body:
- Negatively affects detoxification systems, antioxidant systems and glucose metabolism
- Lowers cellular energy production
- Nervous system excitation
- Muscle rigidity or spasms. As we age, more and more calcium remains trapped in the muscles and these become more or less permanently contracted, leading to increasing muscle tension and spasms;
- CVD / Stroke / Hypertension / Heart attack/Vascular degeneration. As a consequence of contracting/constricting blood vessels and hardening/rigidity of the arteries when calcium deposits in artery walls (arteriosclerosis). This in turn leads to restricted blood flow causing high blood pressure and inelastic vessels, which may easily rupture causing strokes. Calcium is a component of arterial lesions and is of course involved in calcification in heart valve. Countries with the highest calcium to magnesium ratios in soil and water have the highest incidence of cardiovascular disease (Australia tops the list).
- Osteoporosis
- Cancer
- Wrinkled skin
- Calcification of soft tissue
- Arthritis – in the joints and connective tissue
- Muscle / Joint inflexibility -contributes to arthritic deformations in later years
- Fibromyalgia
- Migraine – excess calcium can stimulate muscular layer cells of temporal arteries over the temples causing migraine;
- Asthma -excess calcium constricts the smooth muscle surrounding the small airways of the lung, causing restricted breathing and asthma;
- Bone spurs – from abnormal calcium crystals in bones;
- Fusions in skeletal components (E.g. vertebrae);
- Kidney stones
- High blood calcium
- GI tract disorders
- Chronic fatigue syndrome (CFS);
- Dental problems – cavities
- Cataracts
- Senility from calcification of neurons in brain;
- Depression and other mood disorders
- VLDL triglyceride increase
- General mineral imbalances. incl. magnesium, zinc, iron and phosphorus;
- Interfere with vitamin D activity -which has many health-protecting functions (especially important in cancer prevention)
- Acceleration of aging process

Magnesium has a calming effect on the nervous system
The neurological effects of magnesium
Mg++ (at high extracellular concentration) reduces electrical excitation by diminishing synaptic transmission. Achieved via blocking release of acetylcholine into the synaptic gap between neurons.
Mg++ reduces neuronal excitability in its role as a natural “Calcium Channel Blocker”. Mg++ ions (and other ++ cations at mM concentrations) decrease the activation of certain membrane-bound, voltage-gated calcium channels, and in limiting the influx of extracellular calcium++ into the neuronal cytosol, thereby reduce neuronal excitability.
Mg++ reduces pain. By blocking N-methyl-D-aspartate (NMDA) glutamate receptors, an excitatory neurotransmitter of the central nervous system; prevents ion-flow at typical neuronal resting potentials. Mayer, 1987
Mg is a “calcium channel blocker” in neurons
Mg++ at high extracellular concentrations blocks Ca++influx and diminishes synaptic transmission. Dodge, 1967
If Mg levels are low, nerves lose control over muscle activity, respiration and mental processes. Nerve cells (neurons) need sufficient magnesium to give or receive messages, without which they become excitable and highly reactive. This causes the person to become highly sensitive and highly nervous, possibly resulting in:
- Nervous fatigue
- Irritability
- Restlessness
- Disorientation
- Tics / Twitches
- Hypersensitivity E.g. sharp muscle reaction to an unexpected loud noise.
- Anxiety
- Irregular heartbeat
- Tremors
- Muscle spasms
- Confusion
These effects manifest themselves in several well-known neurological /neuromuscular conditions. Including sleep disorders, the “shakes” in alcoholics, Parkinson’s disease, migraines, pre-eclampsia, suicidal tendencies, epileptic seizures, pain, SIDS, CFS and psychiatric disorders:
Magnesium for Neurological / Neuromuscular conditions
Mg is cofactor for ATPase pumps / ATP production
ATP-producing mitochondria are highly concentrated in cells of the brain and central nervous system. Since neurotransmission has an avid need for ATP energy

Magnesium powers cell "Battery"
First some background on the cell battery and its transmembrane pumps
What is the cell “Battery”? The Cell “Battery” is the voltage difference across the plasma cell membrane (a.k.a. the transmembrane potential). Typically -70 mV in a healthy cell, with outer membrane being more positive than inside. The charge difference is determined by an imbalance of mineral ions, such as K+, Na+, Ca++ and H+, separated on either side of the membrane.
Body’s energy currency ATP and the protein enzyme ATPase. ATP (adenosine triphosphate) is produced by ALL of our cells (by cell mitochondria) to store energy (as phosphate bonds) to be used for work in the cell – in an aqueous medium, energy is released from ATP by the enzyme ATPase, which breaks down (hydrolizes) the phosphate bonds in ATP (into ADP plus a free phosphate ion). Without the ATPase enzyme, ATP’s stored energy can not be extracted.
Some ATPases are transmembrane pumps that provide an active transport channel for ions, such as H+, Na+, K+ , Ca2+ across a cell membrane – transmembrane ATPases (pumps) are integral proteins of the membrane which make it possible for the solute ions /molecules to diffuse across the membrane against their concentration gradient (i.e. Via active transport) using energy released by the hydrolysis/ break down of ATP.
The most significant pumps are:
- Sodium/Potassium pump (Na+/K+– ATPase) – found in the plasma membrane of all animal cells; used to maintain the transmembrane potential (voltage) by powering movement of sodium ions (out of the cell) and potassium ions (into the cell) across the membrane against their gradient
- Bicarbonate pump (HCO3 –ATP ase) – present in parietal cell membranes in stomach; used to acidfy the stomach
- Calcium pump (Ca2+–ATP ase) –
Magnesium is necessary to power the transmembrane transport pumps
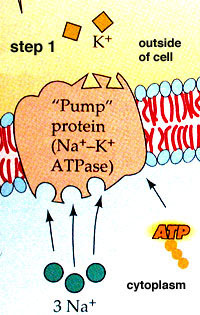
- ATP must be bound to a magnesium ion to be biologically active. Investigations of the Na/K–ATPase established that Mg2+ is an essential cofactor for activation of enzymatic ATP hydrolysis to release cellular energy from ATP.
ATP + Mg 2+ <–> MgATP2-
MgATP2-. binds to the ATPase enzyme (on the cytoplasmic side of the membrane, without magnesium being transported through the cell membrane) and remains bound throughout the reaction cycle at least until after the release of phosphate. Most ATPase enzymes break down MgATP2- .
- Magnesium also REGULATES ATP production – experimental evidence collected through the years confirms that Mg2+ ions have a regulatory effect on ion transport by interacting with the cytoplasmic side of the ion pump. Apell et al, 2017, Fukushima & Post, 1978, Mildvan AS, 1987
Effects of having insufficient magnesium available for transmembrane pumps
Lack of magnesium allows cells to swell. Gradient created by Na/K pump is used to expel excess water from cell to prevent it from swelling;
Lack of magnesium reduces cell “battery” voltage. The Magnesium-dependent Na/K pumps maintain appropriate intracellular/extracellular potassium / sodium ion concentrations
- The Na/K pumps are opened or closed when stimulated by a change in the cell “battery” voltage – the opening of the Na/K pump generates an inward current that affects the membrane potential itself (creating a reinforcing positive loop).
- “No gas (i.e. magnesium), No Go !”- a malfunctioning Na/K pump due to a lack of Mg-dependent ATP reduces the cell “battery” voltage and mitochondrial ATP energy production in the cell, which further negatively affects Na/K pumps. A rat study found that a magnesium deficiency decreased Na/K pump activity in heart cell membranes. Effects of dietary magnesium on sodium-potassium pump action in the heart of rats.
Chronic magnesium deficiency leads to intracellular calcification
Magnesium is a natural calcium channel blocker, responsible for muscle relaxation to counter calcium’s contraction
Na gradient generated by Magnesium-dependent Na/K pumps is used by cell membrane and endoplasmic reticulum membrane calcium pumps (Na+ – Ca2+ translocators, found in smooth and striated muscle cells) to regulate calcium concentration in the cell’s cytoplasm (i.e. inside the cell). Calcium usually enters cells for an excitory action E.g. a muscle contraction or nerve impulse. Once its job is done, magnesium empowers calcium pumps to flush calcium out of the cell against its gradient, or into intracellular calcium stores, such as the sarcoplasmic reticulum of muscle cells or mitochondria of all cells). A deficiency of magnesium leads to calcium accumulation inside cells(called calcification), which can result in over excitation in nerve and muscle cells. This not only affects “movement muscle” contractions (seen as spasms), but also affects heart and arterial contractions.
Familiar health consequences ensue as calcium accumulates inside cells (calcification). Including: arteriosclerosis/CVD, cancer, hypertension, arrhythmias, angina pectoris, neurodegenerative diseases, muscle/joint pain and stiffness, muscle spasms/ twitching, tension / migraine headaches, painful menstrual ramping, cataracts, bone spurs, and on and on . . . Effects of calcification include:
- Build up of calcium in soft tissues. Magnesium prevents soft tissue calcification
- Inability to relax muscles. Muscle contraction depends on a calcium ion concentration bout 10,000 times higher than its resting concentration inside cells, which is accomplished by pumping calcium in via the membrane calcium pumps and by using calcium pumps in the sarcoplasmic reticulum (SR) (a storage depot for calcium, which is a special type of endoplasmic reticulum found in smooth and striated muscle that sequesters then releases calcium when the muscle is stimulated to contract). Muscle relaxation occurs when calcium is quickly returned from whence it came, which may not occur effectively with a magnesium deficiency. Magnesium relaxes muscles
- Protein/Lipid synthesis and carbohydrate/steroid metabolism. In its role in powering the endoplasmic reticulum membrane calcium pumps, Mg serves to regulate calcium concentrations in the cytoplasm by stimulating sequestration of calcium into the cell’s endoplasmic reticula, which are responsible for:
- Synthesis of proteins / triglycerides / phospholipids / steroids;
- Metabolism of carbohydrates / steroids;

Magnesium maintains cell membrane integrity
- Magnesium maintains membrane permeability, flexibility and stability by “wearing” several of its different “hats”:
- Affecting >300 enzymes
- Aiding ion transport across cell membranes
- Being involved in fatty acid and phospholipid metabolism;
- Prevents Infection of Cell. Magnesium deficiency would lead to a weakening of the cell membrane, thereby setting the stage for infection of the cell. Note that evidence is building to support the theory that such infections are involved in cancer.
- Membrane integrity affects the ability of cells to prevent calcium from accumulating inside the cells. A compromised cell membrane (or low ATP production) makes it difficult for the cell to maintain the normal concentration gradient of 10,000 times more calcium outside of cells than inside; when this happens there is increased intracellular calcium.
- In vivo, magnesium deficiency increases membrane fluidity and permeability. Heaton et al, 1989

Magnesium needed for cellular and food metabolism
Magnesium is deeply and intrinsically woven into cellular metabolism:
- Mg2+ -dependent enzymes appear in virtually every metabolic pathway
- Specific binding of Mg2+to biological membranes is frequently observed
- Mg2+ is used as a signaling molecule
- Much of nucleic acid biochemistry requires Mg2+. Including all reactions which require release of energy from ATP
Magnesium needed to metabolize food
- Magnesium is necessary for the metabolism of carbohydrates, fats and amino acids, and many other biologically active nutrients and substances. Including calcium, potassium, phosphorus, zinc, copper, sodium, lead, cadmium, hydrochloric acid (HCl), ACETYLCHOLINE, and NITRIC OXIDE (NO).
- Magnesium transports many substances (E.g. minerals, mineral electrolytes, hormones, and neurotransmitters) into and out of cell. Via its role in maintaining the cell “battery” level

Magnesium detoxifies cells of toxic metals and other toxins
Magnesium protects cells from toxic metals
Magnesium protects cells from aluminum, mercury, lead, cadmium and nickel prevalent in today’s environment. Toxic metal bombardment is a well-known challenge of our modern day. A consequence of magnesium deficiency (or relative overabundance of calcium) is lower cell membrane “batteries”(and thus loss of pumping capacity), such that toxic metals are less effectively removed from, and thus accumulate in cells. Magnesium (along with other minerals, such as zinc) is vital for phase I detoxification and is particularly invaluable for dislodging toxic metals from the body
- Evidence is mounting that low magnesium levels allow the toxic metal deposition in the brain tissue that precedes Parkinson´s disease, multiple sclerosis (MS) and Alzheimer´s
- Low total body magnesium could also be a major contributor to heavy metal toxicity in children, a factor involved in learning disorderss
Cellular detoxification
Magnesium (and other alkaline minerals) are utilized by the body for detoxification
- Used to process cellular debris
- Counteracts elevated uric acid production when combating a detox reaction. If in short supply, magnesium is taken from bones
Magnesium is one of the most commonly deficient nutrients in the chemically toxic individual. These individuals are observed to excrete high amounts of magnesium in urine, causing low magnesium levels in body
The ability to detox a particular toxin varies upon an individual’s magnesium level. According to Dr. Frederica P. Perera, Professor of Environmental Health Sciences and Director of the Columbia Center for Children’s Environmental Health, who recognizes that although there can be a 500-fold difference in the ability of each person to detoxify a particular toxin, a main determining factor is an individual’s magnesium level,
Magnesium protects the cell from aluminum, mercury, lead, cadmium, beryllium and nickel

Magnesium has a major role in heart health / blood pressure
All the usual risk factors for heart disease can be the result of low magnesium status.
E.g. hypertension, high total cholesterol, low HDL cholesterol, high LDL cholesterol, high homocysteine, and high C-reactive protein. Recent studies show that high anxiety and depression (symptoms of human magnesium deficiency) can predict heart disease even more than the traditional risk factors.
As cofactor in Na/K-ATPase pumps, magnesium provides ATP energy for heart muscle cells. These have an avid and constant need for energy.
Mg levels affect cardiac excitability, contraction, and conduction. Intra- and extra-cellular magnesium levels playan important role via their regulatory effects on intracellular calcium movement in heart muscle cells;
Chronic high blood pressure can be caused both directly and indirectly by a magnesium deficiency
Low cellular magnesium impedes a healthy sodium to potassium ratio. This is necessary for normal blood pressure.
Low magnesium : High calcium in blood vessel muscle cells cause them to contract, resulting in high blood pressure. Mg levels determine vasoconstriction or vasodilation via its powerful role in calcium cycling in smooth muscle of blood vessels:
- Higher magnesium levels inside muscle cells produce a relaxing or vasodilating effect
- Low Mg concentrations inside muscle cells is vasconstricting. A potential cause of hypertension;
Magnesium protects the heart from the negative effects of excess calcium. Mg blocks calcium entry into cardiac (heart) cells and vascular smooth muscle cells, reducing vascular resistance and naturally lowering blood pressure.
Magnesium may act as an anti-arrhythmic agent
Magnesium limits intracellular calcium overload triggered during myocardial ischemia This may be a cause of ventricular arrhythmia; Deranged intra- and extracellular concentrations of magnesium , calcium and potassium can manifest as cardiac arrhythmia
Magnesium deficiency has been implicated and documented in humans. In atrial fibrillation, supraventricular tachycardia, torsade de pointes, ventricular ectopy, ventricular tachycardias, and toxic digitalis arrhythmias. Whang R; Magnesium deficiency -pathogenesis, prevalence, and clinical applications, Am J Med 82:24, 1987
Magnesium has an antioxidant role
Magnesium has an antioxidant role protecting against production of inflammatory cytokines and ROS. Involved with many degenerative diseases
- Cardiac muscle necrosis and lesions were demonstrated in animals fed magnesium-deficient diets. Necrosis/lesions were consequential to ROS originating from activation of immune system cells. Weglicki et al, 1996; Weglicki et al, 1992; Weglicki et al, 1994
Increased susceptibility to ischemic / reperfusion injury of heart shown in animals fed Mg -deficient diets. Reperfusion injuryis tissuedamage caused by returning bloodsupply after a period ofischemia (restricted blood supply), which results in inflammation and oxidative damage rather than restoration of normal function. Weglicki et al, 1994

Magnesium for hormonal balance (crucial to maintaining health)
Magnesium is needed for cholesterol synthesis (precursor to sex steroid hormones)
Cholesterol is a prerequisite for all the steroid hormones produced in the adrenal cortex. Such as:
- ALDOSTERONE. Regulates body’s water and mineral balance, including magnesium
- DHEA. Protects the entire body against the aging process; prohormone for all the sex steroid hormones. E.g. Estrogens, PROGESTERONE, TESTOSTERONE; magnesium deficiency is directly related to reduced DHEA.
- CORTISOL. Released in response to stress and low blood sugar;
Magnesium status controls uptake and release of many hormones, nutrients and NEUROTRANSMITTERS
This was concluded by recent research in France and several other European countries. It accomplishes this via its role in maintaining a healthy cell membrane potential (Cell “battery” level).
Magnesium is crucial to the transmission of:
- Hormones: E.g. INSULIN, thyroid hormones, estrogens, TESTOSTERONE, DHEA;
- NEUROTRANSMITTERS: E.g. DOPAMINE, catecholamines, SEROTONIN, GABA;

Magnesium needed to regulate blood sugar
Intracellular enzyme tyrosine kinase needs magnesium to enable INSULIN to regulate blood sugar. And so prevent blood sugar spikes or crashes
Magnesium has a major role in preventing high blood pressure. Which could otherwise lead to INSULIN resistance (IR) i.e. INSULIN being ineffective

Over 300 enzymes need magnesium to function
Mg2+ –dependent enzymes appear in virtually every metabolic pathway
- Magnesium serves as “gatekeeper” to stop calcium entering into and activating a nerve – with Mg deficiency, the nerve can become over-activated, which can cause muscle tension, soreness, spasms, cramps and fatigue, migraine, and spastic lower esophageal and pyloric sphincter function leading to GERD;
- Keeps heart rhythm steady. The heart is a muscle – research suggests that cardiac/heart muscle is more sensitive to magnesium intake than skeletal muscle.
- Supports a healthy immune system – fighting infection
- Helps regulate blood sugar levels. Intracellular enzyme tyrosine kinase needs Mg to allow insulin to lower blood sugar
- Key involvement in cellular ATP energy production
- Converts Vitamin D to CALCITRIOL (its active form). Crucial in many health functions
- Other enzymes that rely on Mg – creatine-kinase activation, adenylate-cyclase, and sodium-potassium-ATPase
- Helps maintain normal muscle and nerve function (works together with calcium). Mg affects cell mechanisms controlling muscle and nerve cell activity.
- Orchestrates the electric current that sparks through the body’s miles of nerves
- Promotes proper/strong formation of bones and teeth
- Promotes normal blood pressure. Stimulates production of prostacyclins and nitric oxide (NO) -both potent artery-relaxing agents.
- Promotes normal bowel function
- Involved in protein synthesis
- Helps transport other minerals across cell membranes

Magnesium needed for synthesis of glutathione (the major "in-house" antioxidant)
Glutathione, a primary antioxidant and detoxifier of the cell cytoplasm, requires Mg for its synthesis – a major antioxidant produced in the body, glutathione detoxifies intracellular toxins, such as heavy metals, and chemicals inhaled from cigarette smoke and car exhausts. Heavy metal presence increases oxidant damage to cells, which has long been recognized as a factor in many degenerative diseases.
- Glutathione provides a highly reactive “bulls-eye”for radicals
- Glutathione helps red blood cells carry oxygen
- Glutathione is needed for the creation and maintenance of T-cell lymphocytes -the immune system’s frontline defense against infection
- Glutathione is one of the few antioxidant molecules known to neutralize mercury
According to Dr. Russell Blaylock, low magnesium is associated with glutathione depletion and dramatic increases in oxidant generation. Without the chelating and antioxidant presence of glutathione, cells weaken under oxidant damage, setting the stage for cellular damage and infection, likely factors in cancer, and any of the other degenerative disease seen today.
For the technobuffs. Glutathione synthetase requires y-glutamyl cysteine, glycine, ATP, and magnesium ions to form glutathione. In magnesium deficiency, the level of enzyme y-glutamyl transpeptidase is lowered.

Magnesium is a muscle relaxant
Muscles 101
The function of muscles is to produce force and cause motion. Either as locomotion of the organism itself or movement of internal organs.
There are three classifications:
- Skeletal muscle. Anchored by tendons to bone to effect skeletal movement, such as locomotion or posture
- Cardiac muscle. Found only in the heart; responsible for pumping blood; similar to skeletal muscle
- Smooth muscle. Found within walls of organs/structures such as esophagus, stomach, intestines, bronchi, uterus, urethra, bladder, blood vessels; responsible for sustained contractions.
When referring to the muscular system, “contraction” means that muscle fibers generate tension with the help of motor neurons. We use our muscles by selectively contracting them via:
- Voluntary (conscious) contraction of skeletal muscle (controlled by action potential signals from the central nervous system) .
E.g. movement of quadriceps muscle to kick a ball, or eye movement occurs as a result of conscious effort originating in the brain. The brain sends action potential signals through the nervous system to the motor neuron that innervates several muscle fibers. In the case of some reflexes, the signal to contract can originate in the spinal cord through a feedback loop with the brain’s grey matter.
- Involuntary (without conscious thought) contraction of cardiac or smooth muscle (non-conscious brain activity or stimuli from the body to the muscle). Necessary for survival.
E.g. contraction of the heart muscle for heartbeat, peristalsis (pushes food through GI tract)
Calcium pumps move calcium powered by ATP produced by magnesium-dependent Na/K pumps
Plasma membrane Ca2+-ATPase (PMCA) pump moves calcium into and out of all eukaryote (nucleus-containing) cells. Ca2+ is an important second messenger (relays messages from membrane receptors to intracellular targets), so intracellular levels must be maintained at low concentrations to prevent noise, in order to have messages delivered properly (called cell signaling).
- PMCA and the sodium calcium exchanger (NCX) are the main regulators of intracellular Ca2+ concentrations. Since PMCA transports Ca2+ into the extracellular space, it is also an important regulator of the calcium concentration in the extracellular space.
- The PMCA pumps are powered by the hydrolysis of magnesium-dependent ATP (One Ca2+ ion removed for each molecule of ATP hydrolysed). PMCA binds tightly to (has high affinity for) Ca2+ ions but does not remove Ca2+ at a very fast rate, and is well-suited for maintaining Ca2+ at its normally very low levels. In contrast, NCX has a low affinity, but a high capacity and is thus better suited for removing large amounts of Ca2+ quickly, as is needed in neurons after an action potential.
Sarcoplasmic reticulum Ca2+-ATPase (SERCA) pump. in muscle cells, the SERCA pump pumps calcium previously released from cell cytoplasm, back into the sarcoplasmic reticulum, a cell organelle that acts as a storage depot for calcium inside the muscle cell.
- The SERCA pumps are powered by the hydrolysis of Mg-dependent ATP
Magnesium roles in contraction/relaxation of muscle
The Mg-dependent Na/K pumps maintain appropriate intracellular/extracellular potassium/sodium ion concentrations
The electrical action potential signal in a muscle cell to initiate a contraction involves complex movement of sodium and calcium ions into and potassium ions out of the muscle cell to propogate an action potential and depolarize the cell. There must then be a rapid restoral of the ions against their electrochemical gradients for the cell to repolarize and be ready for the next action potential.
Magnesium – Cell “Battery” / ATP Production
Muscle contraction (The Calcium Cycle) occurs in response to a nerve’s electrical action potential stimulus. Contraction of cardiac and smooth muscle requires rapid shifting of intracellular calcium ions to maintain appropriate gradients; a muscle contraction is initiated when intracellular calcium is released from the sarcoplasmic reticulum or calcium enters the cell from the outside.
Mg++ levels inside and outside the cell have an important role in the intracellular calcium cycle in muscle cells
Muscle relaxation requires intracellular calcium to be quickly pumped back into the sarcoplasmic reticulum – via magnesium-dependent SERCA pumps and is pumped out of the cell via PMCA pumps after the calcium cycle is completed.
Mg-dependent Na/K-ATPase pumps are vital for production of mitochondrial ATP energy needed to enable SERCA pumps to quickly shunt Ca++ back into the sarcoplasmic reticulum and PMCA pumps to pump Ca++ out of the cell
Magnesium is a “Calcium Channel Blocker” (for some cell membrane-bound calcium channels). Magnesium++ serves as an important gating mechanism limiting the influx of extracellular calcium into the cytosol via PMCA pumps. Magnesium is thus similar to calcium channel blocker drugs, which lower blood pressure by blocking calcium entry into heart and smooth muscle cells of blood vessels.
Magnesium limits the influx of extracellular calcium into the cell cytoplasm
Magnesium effects on heart health
Magnesium is a muscle relaxant in skeletal muscle contractions
“Do move a muscle”101
- The neuromuscular system is the combination of the nervous system and muscles, working together to permit movement. The brain controls the movements of skeletal (voluntary) muscles via specialized nerve cells (neurons).
- Process to move a body part. A message (an action potential) is sent to upper motor neurons, which have long tails (axons) that go into and through the brain, and into the spinal cord, where they connect with lower motor neurons. At the spinal cord, ~50-200 lower motor neurons in the spinal cord send their axons via nerves in the arms and legs directly to the muscle they control.
Each lower motor neuron is subdivided into many tiny branches. The tip of each branch is called a presynaptic terminal. The connection between the tip of the nerve and the muscle is called the neuromuscular junction. - The neurotransmitter ACETYLCHOLINE triggers skeletal muscle contraction. The electrical signal from the brain (action potential) travels down the nerves (neurons) and prompts the release of the chemical ACETYLCHOLINE from the presynaptic terminals. This chemical is picked up by special sensors (receptors) in the muscle tissue. If enough receptors are stimulated by ACETYLCHOLINE your muscles will contract. For skeletal muscles, the force exerted by the muscle is controlled by varying the frequency at which action potentials are sent to muscle fibers.
Magnesium role in muscle tension and spasms
- Magnesium depletion leads to increased neuronal excitability and enhanced neuromuscular transmission. The opposite effect occurs with magnesium excess. An example of acute CNS magnesium deficiency is found in cattle with “grass staggers”(tetany), leading to severe muscle seizure and even death. In humans, a chronic magnesium deficit is implicated in neurological and neuromuscular conditions, such as migraine, CFS, and many other sleep and psychiatric disorders.
- A decrease in neuronal magnesium concentration is postulated to increase calcium binding to prejunctional ACETYLCHOLINE vesicles. Increasing release of ACETYLCHOLINE into the neuromuscular cleft and so increasing muscle contractions.
- With a relative deficiency of magnesium to calcium, calcium remains trapped in the muscle cells. Over time, if the relative deficiency continues (as often occurs in aging), the muscles become more or less permanently contracted, leading to increasing muscle tension and spasms.

Magnesium regulates calcium
Adequate magnesium is essential for calcium absorption and metabolism
Calcium (Ca) / Magnesium (Mg) – The classic ying/yang pair
Neither magnesium or calcium can act without eliciting a reaction from the other. Biochemically, magnesium and calcium have complementary and antagonistic roles to each other:
- Magnesium has a pivotal role in energy production and many cellular metabolic processes
- Calcium is more concerned with structure strength (in bones and teeth) and movement (neuromuscular).
Inside the cell. magnesium is a cofactor with ATP providing power for the intracellular pumps. E.g. the important Cell “Battery” Pumps (Na+/K+-ATPase), bicarbonate pumps (HCO3- ATPase), and calcium pumps (Ca2+-ATPase) all need Mg2+ATP to maintain effective ionic gradients within and outside the cell. Magnesium is the second-most abundant positive ion (cation) inside the cell, but most is bound to molecules that regulate energy production, storage and utilization. Magnesium is required in the mitochondrial respiration cycle (during oxidative phosphorylation and anaerobic metabolism of glucose);
Magnesium (Mg) /Calcium (Ca) Interplay
- Magnesium keeps calcium dissolved in bloodstream, heart, brain, kidneys/urine, and in all the tissues in your body. Try crushing a calcium pill in 1oz water and watch how slowly adding a crushed magnesium pill enables the calcium to dissolve.
- Prevents kidney stone formation. Magnesium increases calcium solubility in urine and Mg supplementation has demonstrated a significant reduction in recurrence of kidney stones.
- An insufficiency of magnesium to keep calcium dissolved can result in muscle spasms, fibromyalgia, hardening of the arteries and more.
- Magnesium prevents soft tissue calcification. The higher the calcium level and the lower the magnesium level in the extra-cellular fluid, the harder it is for cells to pump the calcium out. Insufficient magnesium to ensure smooth running of the calcium pumps can result in calcification of soft tissues responsible for a slew of familiar health problems
- Activity of many enzymes depend on a sufficient amount of intracellular magnesium. Detrimentally affected by even small increases in levels of intracellular calcium.
- Growth of cells, cell division, and parts of metabolism depend on magnesium availability. This can be compromised if excess calcium is present.
- Magnesium is essential for calcium absorption and metabolism. Conversely, several studies report that increased calcium intake significantly reduces magnesium uptake and utilization;
- Magnesium maintains proper blood calcium levels
- Magnesium is nature’s “Calcium Channel Blocker” in nerve cells. Calcium enters nerve cells through calcium channels carefully guarded by magnesium, which allows just enough calcium through to create the necessary electrical transmission along the nerve cell, and then once the job is completed, immediately helps to eject the calcium.
- Calcium is needed to contract a muscle and magnesium is needed to relax it. We use our muscles by selectively contracting them. Muscle contraction is triggered by calcium ions flowing into muscle cells. To relax the muscle calcium is pumped out again. Problems, such as muscle spasms, occur when calcium to magnesium ratio becomes too high, usually because of a magnesium deficiency.
- Magnesium is needed to maintain the cell “battery” providing the power for calcium pumps (Ca2+-ATPases)to pump calcium out of cells. Most of the enzymes (E.g. ATPase) involved in mitochondrial ATP production require magnesium. Up to 30% of cellular energy is used to pump calcium out of the cells;
Magnesium protects against high calcium intake linked to higher risk of prostate cancer. A 1998 Harvard School of Public Health study of 47,781 men found those consuming 1,500 – 2000 mg of calcium per day had about double the risk of being diagnosed with metastatic prostate cancer as those getting 500 mg per day or less. Those consuming > 2,000 mg had over four times the risk of developing metastatic prostate cancer as those taking in less than 500 mg.
Commonly today, a high calcium intake is detrimentally out of proportion with a low magnesium intake
- Calcium and magnesium need to be consumed or supplemented in around a 1:1 or 1:2 ratio. Previously thought needed at 4:1 or 2:1, more recent indications suggest magnesium intake should be at least on par with calcium to facilitate assimilation of calcium.
- Current research on the Paleolithic or caveman diets show that the ratio of their diet was 1:1. Eades M, Eades A, The Protein Power Lifeplan, Warner Books, New York, 1999
- Unfortunately, researchers estimate that the Calcium to Magnesium intake ratio is approaching an all time high that favors calcium 6:1. A result of excessive increases in calcium intake, while magnesium intake has decreased or remained unchanged. Calcium is typically coming from a dietary excess of milk and other dairy products and high calcium supplementation
Magnesium (Mg) /Calcium (Ca) ratio changes with age
- Intracellular calcium to magnesium ratio increases with age. Found a study of 103 subjects of various ages measured intracellular levels of magnesium and calcium in red blood cells drawn at 9 AM. Barbagallo et al, 2000
- High calcium to magnesium ratio is clearly involved in hypertension / atherosclerosis and Non-Insulin dependent Diabetes Mellitus (NIDDM) – subjects with hypertension or NIDDM had significantly higher levels of intracellular calcium and lower levels of intracellular magnesium—even worse than the levels seen in older normal subjects (see Figs. 4 and 5).
- Neither age, hypertension or diabetes had any effect on serum (liquid portion of blood, minus the red and white blood cells) concentrations of calcium and magnesium. Only about 1% of magnesium is extracellular, thus serum magnesium levels do not accurately reflect intracellular magnesium content. Furthermore, only 10-15% of intracellular magnesium is in the free, active form. Gupta et al,
- Younger normal subjects had higher levels of intracellular magnesium, and lower levels of intracellular calcium (Figs. 1 and 2) than the normal older subjects
| Subjects | Healthy | Hypertensive | Non-INSULIN dependent diabetes mellitus |
| <65 yrs | 26 | 30 | 15 |
| >65 yrs | 11 | 9 | 12 |
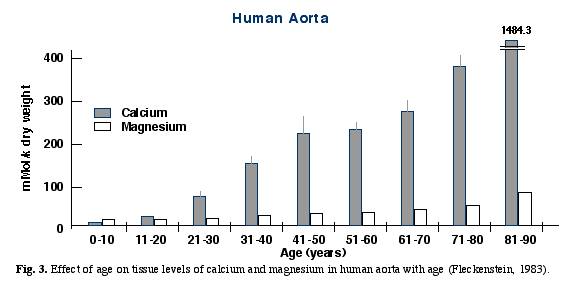
- Fleckenstein found similar changes in tissue calcium and magnesium levels in the aortas of humans who died at various ages. Fleckenstein demonstrated the progressive accumulation of calcium in arterial tissue, resulting in a shift of the calcium /magnesium ratio in favor of calcium (Fig. 3). Fleckenstein A, 1983

References
Barbagallo, M., Gupta, R.K., Dominguez, L.J., and Resnick, L.M. (2000) Cellular ionic alterations with age: Relation to hypertension and diabetes. J American Geriatrics Society. 48: 1111-1116. PubMed
Dae Jung Kim et al (publ. online Aug 31, 2011 ) Magnesium Intake in Relation to Systemic Inflammation, Insulin Resistance, and the Incidence of Diabetes. Diabetes Care, PubMed
Dodge Jr FA, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol 1967; 193: 419-432. PubMed
Fleckenstein, A. Calcium Antagonism in Heart and Smooth Muscle, John Wiley & Sons, New York, 1983.
Gupta, R.K., Gupta, P., Yushok, W.D., Rose, Z.B. Measurement of the dissociation constant of magnesium ATP of 31P-NMR and optical absorbance spectroscopy.
HEATON H.W., TONGYAI S. & RAYSSIGUIER Y. (1989) : Membrane function in magnesium deficiency. In: (Ref. 2), 27-33.
King DE, Mainous AG 3rd, Geesey ME, Woolson RF (2005 Jun) Dietary magnesium and C- reactive protein levels. J Am Coll Nutr. 24(3):166- 71. Pubmed
Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol 1987; 394: 501-527.PubMed
Weglicki WB et al; Role of free radicals and substance P in magnesium deficiency, Cardiovasc Res 31:677, 1996.
Weglecki WB, Philips TM, Pathobiology of magnesium deficiency -a cytokine neurogenic inflammation hypothesis, Am J. Physiology, 263:R734, 1992.
Weglicki WB et al, Cytokines, neuropeptides, and reperfusion injury during magnesium deficiency, Ann NY Acad Sci 723:246, 1994.