Why is the body's acid-alkaline balance of prime importance for health?
Over-acidity (called acidosis) of body fluids has become a health crisis
“We are facing the largest health crisis in recorded history”
– Dr. Theodore Baroody, author of “Alkalize or Die“
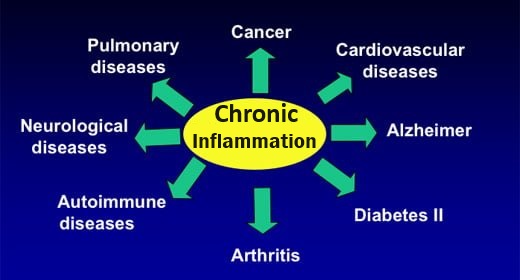
Acidosis in the body is linked to all kinds of illness and disease. It is the result of the typical Western diet and lifestyle. due to, for example, the over-consumption of acid-forming foods compared to alkaline-forming ones and too much stress. (Alkalosis is usually only seen in someone with kidney disease, or one taking too many alkalizing supplements or drugs intended to have an alkalizing effect). The body deals with excess acid by employing its alkalizing mechanisms, which if overused, can eventually deplete the body’s stores of alkaline minerals (e.g. sodium, potassium, magnesium and calcium), paving the way for chronic and degenerative disease.
Levels of acidity or alkalinity in various body fluids are measured by their pH value
Water (H2O) ionizes into hydrogen (H+) and hydroxyl (OH-) ions, which when in equal proportion have a neutral pH value of 7
If there are more H+ ions than OH- ions then the water is said to be acid.
If OH- ions outnumber the H+ ions then the water is alkaline.
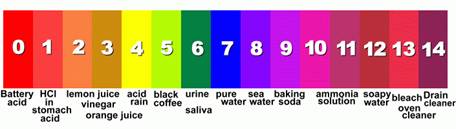
The pH value represents a fluid’s acidity or alkalinity level by indicating its concentration of hydrogen ions (H+) – The most acid pH value is 0, and the most alkaline pH is 14. A fluid’s pH value indicates its concentration of hydrogen ions (H+) and therefore its potential for attracting more hydrogen ions. (a solution with a pH of 0 has no ability to attract hydrogen ions).
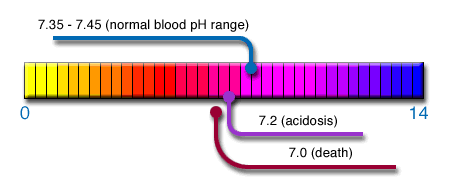
pH values of fluids range from 0 to 14 (i.e. very strong to very weak acids or bases/alkalis)
For optimal body function, the pH level of blood plasma and external and internal cellular fluids must be kept in the narrow pH range of 7.35 – 7.45 – acidosis occurs when this pH drops lower than 7.2 on a regular basis, and has detrimental consequences to health.
Acidosis – What Health problems occur when the body is too acidic?
For health, we need an appropriate balance between acids and alkalis (bases) in blood plasma and body tissues
Chronically imbalanced pH levels in your body will lead to any of the now commonly observed degenerative diseases. Any slight decrease in pH will result in lower oxygen levels in the blood and, therefore, in the cells. A healthy acid–alkaline balance is decisive to the structure and function of proteins, the permeability of membranes, the distribution of electrolytes (charged particles) and the functioning of connective tissue. Examples of physiological processes affected by pH:
- Cleansing and healing processes
- Beating of heart
- Firing of nerves
- Ability to absorb nutrients
- Ability of muscles to contract decreases as body becomes more acidic, while hormones like adrenaline increase.
- Metabolic enzyme activity and chemical reactions
- Oxidation rate in ATP cellular energy production
- Transport proteins that move substances across cell membranes
- Signaling systems that transmit messages between cells or intracellular compartments
- DNA-RNA synthesis
Body processes tend to produce a slightly acid pH – due to acidic end-products, i.e. CO2 and metabolic acids (e.g. sulfuric acids from protein consumption):
- Digestion/Cellular respiration process – which creates energy for a cell to perform its specific task, creates acid end products (waste), which must not be allowed to build up. E.g. lactic acid is created through exercise and can cause pain.
- Breathing;
- Circulation;
- Hormone production
The pH level of the blood plasma and of the external and internal cellular fluids must be kept within a small range of fluctuation under all circumstances. The body is ~70% water-based, and the pH level of the various body fluids inside and outside of cells must be kept within narrow limits in order for many different body processes to be carried out in a controlled way.(E.g. to sustain life, blood plasma pH must be 7.35 -7.45).

How can we increase alkalinity in our bodies?
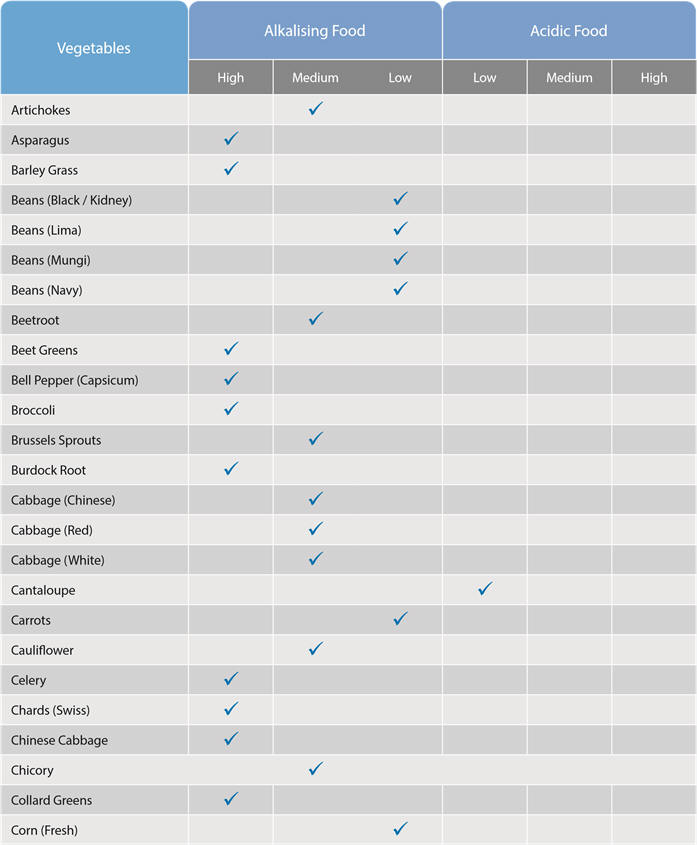
We can alter our body fluid pH levels by what we eat:
These food charts indicate which foods have an acid or alkaline effect on the body
Baking soda can be used as an alkalizing therapy:

Some pH levels of the body fluids
| There are 3 main compartments of body fluids, each separated by selectively permeable cell membranes: | ||
| Internal Cellular Fluid – 2/3 of body fluid is INSIDE cells | (1) Intracellular fluid | |
| Plasma membranes of individual body cells separate intracellular fluid from interstitial fluid | ||
| Extracellular Cellular Fluid (ECF) – 1/3 of body fluids is OUTSIDE cells | (2) Interstitial fluid (also called intercellular fluid) | Bathes outside of cells; ~ 80% of ECF; |
| Endothelial cells separate interstitial fluid from plasma | ||
| (3) Plasma (yellow-colored liquid component of blood, in which blood cells are suspended) | ~~ 20% of ECF | |
| Other ECF fluids | Include lymph, CSF, synovial fluid, humors of the eye, endolymph, perilymph, serous fluids, and glomerular filtrate | |
| Body Area | pH | Comment |
|---|---|---|
| Tissues that deal with the external environment | Acidic | Colon, skin, vagina, stomach, lymph node fluid need an acid pH for proper function |
| Extracellular fluid (Blood plasma and interstitial fluid) | Alkaline | Venous blood; Interstitial pH is ~7.35 (H+ = 40 nmol/l) |
| Intracellular fluid | Slightly alkaline | ~7.0 (H+ = 100 nmol/l); |
| Urine | Acidic | Ideal morning urine pH should be 6.4 – 6.8 |
| Saliva | ~Neutral | First morning saliva pH should be 6.8 – 7.2 |
All other organs and fluids will fluctuate in their range in order to keep the blood at a strict pH between 7.35 and 7.45 (slightly alkaline). This process is called homeostasis. The body makes constant adjustments in tissue and fluid pH to maintain this very narrow pH range in the blood. A normal pH of all tissues and fluids of the body (except the stomach) is slightly alkaline. The stomach pH is much more acid than the intestinal pH because the stomach needs an acid environment (hydrochloric acid) to break down food for digestion. Whereas, the flora (good bacteria) of the intestine need a more alkaline environment to assimilate and process the nutrients from the foods digested by the stomach.

How can we measure the pH of body fluids
We can use pH paper to measure the pH of saliva and/or urine. We can get a pretty good idea of the pH of our body tissues and internal fluids by determining the pH of saliva and urine.
Saliva pH reflects your success in creating an alkaline condition within your body. It should be 6.5-7.5 all day for someone in a healthy state. pH readings should be taken one hour before or two hours after a meal. A pH below 6.0 indicates that you should pay immediate attentionto alkalizing your diet.
Urine pH reflects the pH corrections by the kidney buffer systems. E.g. An alkaline urine simply shows that some alkaline minerals are being discarded by the body.
- People in North America tend to consume too much calcium and sodium andinsufficient magnesium and potassium. This is often reflected in urine tests which show calcium and sodium being excreted while magnesium and potassium are being retained.

Regulatory buffering systems in the body compensate for any excess acid or base
These are the three main systems that buffer acids and bases by functioning in equilibrium with each other:
- Chemical buffer systems. Act within seconds;
- Respiratory compensation. Respiratory center in the brain stem acts within 1-3 minutes; This system is in play when you become “out of breath “after exercise – your muscles produce lactic acid, which is breathed out in the form of CO2 with a stepped up breathingRequire hours to days to effect pH changes
- Renal mechanisms (excretory kidney function).
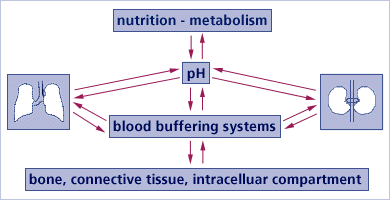
The body’s pH-regulating organs, LUNGS, LIVER and KIDNEYS, remove acidic end products. These would otherwise “corrode” tissues and disrupt cellular activities and functions. For more technical information on how the body buffers excess acid so prevalent today:
MORE TECHNICAL INFORMATION ON PH
The pH scale goes from 0 to 14 and is logarithmic. This means that each step is ten times higher that the previous. (E.g. pH of 4.3 is 10 times more acid than 5.3, and 100 times more acidic than 6.3)
- pH = -log (H+ conc.) On the logarithmic scale the difference between each pH number is a factor of 10.A pH of 5 means 10-5 (i.e. 1/105 ) g/L of H* ions. i.e. 10 x more acidic than a pH of 6 (10-6 g/L)
The STRENGTH of an acid or base is determined by how fast and how much of it dissociates into ions – E.g. A strong acid will have lots of H+ ions, a weak acid not so many.
- Ionization occurs when molecules are “pulled”apart in solution, creating negative and positive ions –E.g. Water (H2O or HOH) is usually very weakly ionized, but certain substances can “pull” at the water to separate it into electrically charged H+ and OH– ions (When these ions are in equal proportions, the pH is a neutral 7). Most organic acids are weak, but mineral acids (e.g. HCl) are much stronger.
- An acid can be neutralized by adding an alkali
| Acidic solution | Contains more H+ ions than OH–ions; | Can donate protons Or Accept electrons/negatively charged ions |
| Alkaline solution | Contains more OH–ions than H+ ions | Can accept protons Or Donate electrons/negatively charged ions. |
Proton – positively charged ion e.g. Hydrogen ion ( H+ ); Electrons /negatively charged ions – carry a negative charge e.g. hydroxyl ion (OH–)