Lp(a) - "The Cholesterol Repair Man" in atherosclerosis

What is Lp(a)?
Lp(a) is an abbreviation for Lipoprotein (a)
The "sticky" form of LDL cholesterol that Drs. Pauling & Rath identified as the primary risk factor
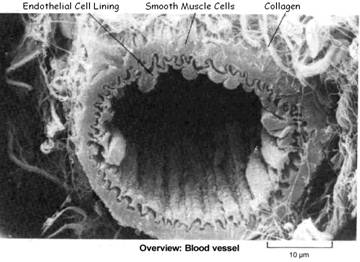
Lp(a) is a variant of LDL (low density lipoprotein) – and like LDL, is manufactured by the liver as a transporter for cholesterol and triglycerides. It is mainly Lp(a) (not LDL) cholesterol that binds to damaged arterial walls to promote the formation of a repair patch, which is known as atherosclerotic plaque.
Plasma Lp(a) levels are:
- One of the best markers for heart disease;
- Controlled by vitamin C
Lp(a) Structure = oxidized LDL + Apo(a)
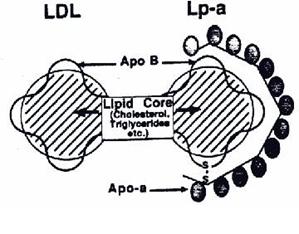
Lp(a) is basically a highly-oxidized LDL cholesterol, with an attached “sticky” apoprotein called Apo(a). Lp(a) (the “a” stands for adhesive) like all lipoproteins is comprised of cholesterol, triglycerides, protein and phospholipids. Similar to LDL cholesterol, Lp(a) is draped with an apoprotein called Apo B100, but in Lp(a), the Apo B100 is chemically bonded to an additional large apoprotein known as Apo(a), which makes Lp(a) behave differently than LDL:
The presence of Apo(a) prevents Apo B100 binding to an LDL receptor
Apo(a) has a sticky, “Velcro” nature, causing it to easily tie up in blood vessels – “Sticky” Apo(a) has amino acid lysine and proline binding sites which it can use as “grappling hooks” to attach to lysine and proline residues exposed in a damaged arterial wall.

Elevated Lp(a) level is the best predictor for cardiovascular disease risk
Lp(a) is involved in the body’s response to injury to the blood vessel wall, which underlies advanced atherosclerotic disease – and is decisively identified as a factor that increases cardiovascular risk primarily in patients in whom other risk factors are also present:
- Lp(a) deposition in the arterial wall was found to correlate with the extent of plaque development – in both the human aorta and the coronary arteries. (Niendorf et al, 1990)
- Patients with vascular disease on average have an Lp(a) level 1.4 times that of healthy controls;
- Patients with high levels of Lp(a) had a 70% higher risk of developing heart disease over a period of 10 years – found British researchers analyzing the findings from 27 studies (Circulation, Sep 5, 2000:102)
- Lp(a) levels rise during early childhood reaching a plateau throughout adult life – Further rises may be seen in post-menopausal women, likely to be related to hormonal changes or the inflammatory effect of increased ferritin (iron) stores when menstruation ceases. (Consider the related benefits of donating blood in menopause). (Mascitelli et al, 2010)
- Lp(a) above 30 mg/dl doubles the risk of CHD and if in addition LDL is elevated, the risk is increased by a factor of 5. (Armstrong et al, 1986) . The risk of CHD also increases if low HDL cholesterol and hypertension are coexistent with elevated Lp(a) – Investigators found that 20% of all thrombo-embolism patients had Lp(a) > 30 mg/dL, compared to only 7% of healthy controls.
- There is no correlation between blood Lp(a) levels and cholesterol levels – in advanced atherosclerosis, Lp(a) is an independent risk factor not dependent on LDL;
i.e. Lp(a) is an indicator of atherosclerosis without hyperlipidemia;A Journal of the AMA Study published in 1996 answered the intriguing question as to why some people with normal cholesterol levels have heart attacks? – This study began with 2191 men showing no signs of CHD. After 15 years they still averaged only a slightly elevated 200 mg/dL cholesterol level, and yet 129 of the men suffered premature heart disease. The significant finding was that these subjects had high levels of Lp(a), even if they had normal HDL and LDL levels.
- In CHD patients with normal lipid levels, the only risk factor for CHD is found to be elevated blood Lp(a) or decreased vitamin C and vitamin E levels. (Rath, 1989)
- Men whose Lp(a) levels were in the highest 20%, had triple the risk of a major coronary event compared to those with lower levels (VonEchardstein et al., 2001)
- Substantial reductions in LDL-C reduce the clinical threat of persistent elevations of Lp(a) – However, common treatments for lowering LDL-C usually have no immediate effect on Lp(a). (Maher et al, 1995)
- Rapid progression of arteriographically-determined CHD has been significantly more common in subjects with Lp(a) levels higher than 25 mg/dL – Approximately 33% of the U.S. population have elevated levels of Lp(a) (>25 mg/dL). (Circulation, 1995)
- Compared to Caucasians, African Americans have higher Lp(a) levels without atherogenic risk – the concentration of Lp(a) in African Americans is from 2-4 times higher than in matched caucasian Americans, although the overall atherogenic risk in the two racial groups appears comparable;
Blood Lp(a) Values
Elevated Lp(a) levels and the reason why Lp(a) infiltrates your arteries can be prevented by ensuring a sufficiency of vitamin C.
When considering CVD risk, the absolute value of blood Lp(a) is not as important as the change in Lp(a) from the norm.
Lp(a) blood concentrations in human beings can vary greatly determined largely by heredity. Utermann G et al, Proceedings of the National Academy of Sciences USA 86, 1989; Utermann G et al, Human Genetics 78, 1988, and to some extent by environmental factors, and especially nutrition (Kostner et al)
- Lp(a) levels can vary from < 0.1 mg/dL to > 100mg/dL between individuals and differ significantly between ethnic groups (Albers et al, 1990; Bovet et al, 1994)
- Both Caucasians and African-Americans have plasma levels of Lp(a) highly dependent on heredity (inversely related to the number of kringle 4 repeats in the apo(a) gene) (Boerwinkle 1992)
The following figures apply to those of European descent:
- The typical range for Lp(a) is 0-30 mg/dL – Lp(a) values > 20-30mg/dl tend to indicate a higher risk for CVD, including atherosclerosis, at least in Caucasians (Bostom et al., 1994) ;
- What should your Lp(a) levels be? – According to Dr. Stephen Byrnes, ND:
- Acceptable serum Lp(a) levels would be <10 mg/dL
- 11-24 mg/dL are borderline high;
- >25 mg/dL are very high. CHD is significantly more common in subjects with Lp(a) levels higher than 25 mg/dl (Circulation, 1995)
~33% of the U.S. population have Lp(a) levels >25 mg/dl
Dr. Linus Pauling stated, “If you have more than 20 mg/dl of Lp(a) in your blood it begins depositing plaques, causing atherosclerosis.”
Lp(a) is seldom tested in the U.S. – and there are currently no international Lp(a) testing standards.
Lp(a) promotes the atherosclerotic process
Lp(a) is released in response to acute injury, infection, or other inflammatory conditions – Other acute-phase reactants (APRs) that are linked to higher CVD risk include C-reactive protein (CRP) and fibrinogen;
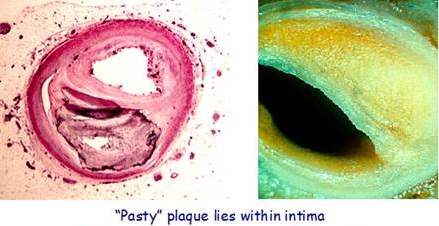
Lp(a) Cholesterol is used to form arterial plaque and promote blood clotting as a “save-your-life” repair mechanism – Lp(a) can generate products that promote atherosclerosis and blood clot (thrombus) formation, as a result of Lp(a) being modified by oxidative events and by the action of enzymes that break down fats and proteins. (Jelakovik et al, 2002). Lp(a) compensates for a decreased rate in collagen formation by creating plaque to repair lesions in the endothelial artery walls, particularly when ascorbate concentration is low. This would have been a great defense for one of our ancestors, after being pierced by a fang or a claw!
Lp(a) accelerates healing of injured blood vessels and promotes other needed cellular-repairs by several mechanisms:
- Prevents the digestion of blood clots on an injured blood vessel – Lp(a) inhibits fibrinolysis (fibrin breakdown) affecting platelet function; ( Lp(a) competes with plasminogen via Apo(a) and increases the activity of inhibitors of plasminogen-I activator);
- Binds to the extracellular matrix of injured vessels, rapidly delivering the cholesterol needed to strengthen the cell wall – by binding to fibrin and other ECM components, it is supposed that Lp(a) compensates for decreased collagen production. However, atherosclerosis would eventually occur if this Lp(a) substitute function were overused.
- Prevents lipid peroxidation;
- Promotes migration and proliferation of media smooth muscle cells – to “beef up” the arterial walls. Lp(a) reduces the activity of transforming growth factor- β (TGF-β)
- Promotes foam Cells – Lp(a) binds to elastin via apo B, resulting in oxidation and facilitated entry into macrophages and their transition into foam cells.
- Inhibits the clearance of chylomicron remnant particles in a transgenic mouse model – recent data have revealed a potential role for Lp(a) in the preferential binding of oxidized phospholipid adducts through one of the kringle motifs in apo(a). (Koschinsky ML. 2006)
Vitamin C ▲ ---> BETTER HEART HEALTH <--- Lp(a) ▼
Blood Lp(a) and Vitamin C (ascorbate) are correlated to wound healing / atherosclerosis (also cancer, diabetes and other health problems)
CVD is decreased by a sufficiency of Vitamin C (ascorbate)
Presence of sufficient vitamin C maintains the integrity of the vessel lining by:
- Protecting against vessel injury – prevents free-radical damage in its role as an antioxidant;
- Participating in vascular repair – enhances the extracellular matrix by increasing collagen synthesis, which leaves the vessel wall smooth and strong.
Lp(a) can be normalized by vitamin C – a 1990 report showed that vitamin C reduces risk for CVD (Passwater)
Ascorbate concentrations ▲ –> Blood levels of LDL ▼ + HDL levels ▲ , and over time, Lp(a) blood levels ▼
Also, large quantities of ascorbate are needed to regulate blood cholesterol levels by converting cholesterol to bile – without enough vitamin C, excess cholesterol and Lp(a) will build up in the bloodstream.
Lp(a) is a "Pinch-hitter" for Vitamin C (ascorbate)
When there is insufficient ascorbate, then Lp(a) is intentionally synthesized – Lp(a) infiltrates the area of injury, together with other lipoproteins and repair substances, to form a “sticky” patch, which is liable to grow into plaque if the substitute function of Lp(a) is overused.
Human and non-human primates are pretty much the only animals that develop atherosclerosis (although rabbits and some other rodents can be forced to develop heart disease when fed a diet loaded with saturated fat and cholesterol, but the disease process is very different from that seen in humans).
Drs. Pauling and Rath proposed that Lp(a) is a surrogate for ascorbate in human and non-human primates (E.g. apes, monkeys, lemurs), the guinea pig and thefruit bat. Detectable amounts of Lp(a) are found almost exclusively in the blood of these species, which have lost the ability to make their own vitamin C, but rarely in the blood of animals that still produce their own vitamin C (the European hedgehog is one exception!).
Dr. Pauling’s hypothesis is that primates evolved Lp(a) to help them heal blood vessels which were more prone to injury because they had been weakened by poor collagen synthesis due to lack of vitamin C – i.e. Lp(a) was a quick survival response to injury for the short and dangerous lives of our paleolithic, hunter/gatherer ancestors;
Vitamin C dosages sufficient to correct our lack of C does not necessarily lower Lp(a) levels, but may simply keep blood vessel walls strong, preventing injuries that require the binding of Lp(a)
A small human trial run by Dr. Rath, people with high Lp(a) levels who supplemented with 9g of ascorbate for 14 weeks experienced an average 27% reduction in Lp(a) levels. However, a larger, controlled trial failed to confirm this result. The larger trial used only half of the dose of vitamin C used in Dr. Rath’s trial, so the result could just be due to a failure to use enough ascorbate. Lp(a) levels, which are mostly determined by your genes, may take a while to return to normal lower levels, or it may be that vitamin C doesn’t actually lower the level of Lp(a). The only conclusively proven ways to lower Lp(a) levels are niacin supplements (which can be taken in the form of inositol hexanicotinate).
If sufficient vitamin C is available to protect the vessel from injury and to participate in vascular repair, the need for Lp(a) is immaterial. Without adequate amounts of vitamin C, Lp(a) is a life-saver.
Guinea pig Experiments – Ascorbate (40 mg / kg of body weight per day) prevented the development of atherosclerotic lesions in the guinea pig and the accumulation of Lp(a) in the arterial wall. An analogous mechanism in humans is suggested because of the similarity between guinea pigs and humans with respect to both the lack of endogenous ascorbate production and the role of Lp(a) in human atherosclerosis. The guinea pig is the only rodent that does not synthesize its own vitamin C. In the 1950s, the Canadian cardiologist Dr. G. C. Willis demonstrated that guinea pigs on a diet lacking saturated fat or cholesterol develop lipid deposits in their arteries (identical to human atherosclerosis), if their diet is also low in vitamin C. Dr. Willis also found that this atherosclerosis could be reversed by high-dose vitamin C supplementation.
Pauling and Rath repeated and expanded Dr. Willis’ work, suggesting that:
The minimum amount of vitamin C required to prevent the development of atherosclerosis in a 70 kg (154#) human, is 2,800 mg of vitamin C / day.
Factors Affecting Lp(a) blood levels
Lp(a) blood concentrations in human beings vary primarily with heredity and with disease states, and are somewhat affected by environmental factors, exercise and nutrition (Kostner et al)
- Heredity (Genes) – ~90% of Lp(a) concentration is under parental genetic regulation. (Lp(a) plasma concentrations are almost exclusively controlled by the apo(a) gene localized on chromosome 6 (in q2.6 – q2.7). Despite this genetic regulation, some metabolic abnormalities may have effects on Lp(a) levels in plasma. Among these, the acute-phase response, hormonal homeostasis, diabetes, liver and renal failure, and defects in the LDL-receptor gene have all been shown to influence the metabolism of this lipoprotein. Black people tend to have higher Lp(a) levels.
Lp(a) increased ▲ ▲ ▲ by:
- Trans Fats – significant increases in Lp(a) levels of subjects consuming diets high in trans fats, but not in those consuming high levels of saturated fats. Dr. Mary Enig, renowned nutritionist, maintains that saturated fats actually lower Lp(a) levels. (Mensink et al, 1992)
- Soy protein – Danish researchers report that dietary soy protein appears to increase Lp(a) levels; (Amer. J. of Clinical Nutrition, 1999)
- 6:00 am to Noon – Japanese researchers reported that morning heart attack victims were found to have significantly higher Lp(a) levels, as the only distinguishable factor compared to another group; they also had a tendency toward hypercoagulation (excess clotting), increasing the risk for developing a life-threatening thrombus or clot. (Fujini et al, 2001)
Lp(a) is reduced ▼ ▼ ▼ by:
- Diet high in vegetables, fruits, and nuts – 24% reduction in Lp(a) levels (Metabolism, 1997)
- Fish consumption – reduced Lp(a) levels, most likely due to its omega-3 fatty acid content Arteriosclerosis Thromb Vascular Biology 1999 In patients consuming large quantities of omega-3-containing walnuts, Lp(a) levels decreased an average of > 6%, as well as an almost equal decrease in LDL cholesterol levels (Zambón et al, 2000)
- Pre-Menopause – the lower prevalence of atherosclerosis in women, compared to men, is possibly accounted for by the protective effect of the female sex hormones and/or menstruation (loss of iron in menstrual blood means less free radicals) – an effect, which is absent after menopause.
- Niacin (nicotinic acid) – can lower Lp(a) levels by around 35-50%; niacin acts as an antioxidant;
- Vitamins C and E and other antioxidants – combat the need for Lp(a) to be oxidized, by countering oxidants and so preventing inflammation;
- Iodine – as an antioxidant having the ability to convert highly reactive and damaging singlet oxygen, to slower-acting triplet oxygen.
Lp(a) unaffected ==== by:
- Statin or fibrate (primarily triglyceride lowering) drug treatments do not generally affect elevated Lp(a) levels
References
Albers et al., 1990;
American Journal of Clinical Nutrition (March 1999) 69:419-425
Armstrong VW et al (1986) Atherosclerosis 62, 249.
Boerwinkle E, et al, (Jul. 1992) Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations, Center for Demographic and Populations Genetics, University of Texas Health Science Center, Houston 77225. PubMed
Bostom et al., 1994
Bovet et al., 1994
Circulation (1995) 91:948-950.
Circulation (September 5, 2000) :102
Fujino et al, 2001
Jelakovik B et al (2002) Lipoprotein (a)–a mysterious factor in atherogenesis
Koschinsky ML, Anuurad E, Boffa MB, Berglund L. (Dec 2006) Lipoprotein(a): a unique risk factor for cardiovascular disease.Clin Lab Med.26(4):751-72. Review PubMed
Kostner GM et al, Treatment of Hyperlipoproteinemia, Raven Press, New York
Maher VMG, Brown G, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. (1995) Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA; 274:1771-4];
Mascitelli L et al (Apr 2010), Menopause, increased iron stores and cholesterol; Maturitas.
Mensink RP, Zock PL, Katan MB, Hornstra G.(1992 Oct) Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J Lipid Res. 33(10):1493-501. PubMed
Niendorf, A., Rath, M., Wolf, K., Peters, S., Arps, H., Beisiegel, U. & Dietel, M. (1990) Virchows Arch. A 417, 105-111;
Passwater RA, How Antioxidant Nutrients Protect Against Heart Disease
Rath, M. et al (1989) Arteriosclerosis 9, 579-592].
VonEchardstein et al., 2001
Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. (2000) Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Annals of Internal Medicine 2000 Online Link