Antioxidants -"Oxidant damage control"


Antioxidants prevent oxidative stress
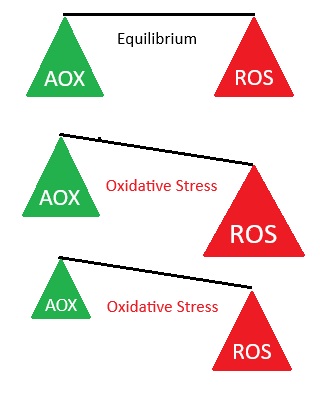
Health is a balancing act between oxidants (mostly ROS) and antioxidants
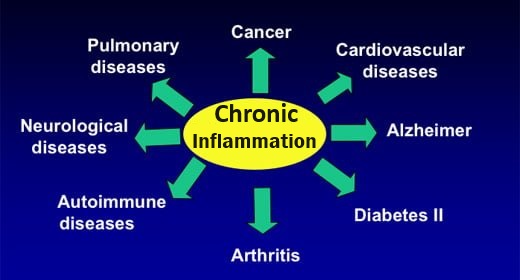
Antioxidants are needed to balance the oxidants produced by and present in the body, preventing cellular damage that initiates many diseases. Normal biological activities of aerobic cells and organisms continuously generate free radicals (oxidants). In humans, the most important are reactive oxygen species (ROS), such as singlet oxygen, hydrogen peroxide , superoxides, and hydroxyl ions, which can inflict cellular damage when they are generated to the extent that they overwhelm the cells’ ROS “damage control” antioxidant systems. Oxidant / antioxidant balance i.e. maintaining oxygen homeostasis (stability), is the major factor in maintaining good cellular function and preventing oxidative stress, which plays a significant role in the progression of many diseases, including diabetes, Alzheimer disease, and atherosclerosis / heart disease.
To prevent damage to cells in the body from excess ROS, we need to:
- Take steps to prevent introducing unwantedROS
- Ensure a balance between the formation of necessary oxidant ROS and the ability of the body’s antioxidant defense to remove them.
ROS:: have a paradoxical presence – although necessary for health, if uncontrolled they can also cause many of today’s familiar diseases:
Life’s Oxygen Paradox – Meet Dr.ROS Jeckyll and Mr.ROS Hyde
Antioxidants sacrifice themselves to preserve your body parts – basically, antioxidants are molecules that ROS, such as free radicals, find “more attractive”than cellular components. Antioxidants readily donate their electrons to prevent free radicals from stealing electrons out of membrane fatty acids, mitochondria, DNA, and elsewhere. After donating an electron, an antioxidant becomes a free radical by definition. Antioxidants in this state are not harmful because they have the ability to accommodate the change in electrons without becoming reactive themselves.
Antioxidants each work in their own unique manner and in their particular area of expertise, but they also complement each other – in a wonderful synergy that effectively protects cells from free radicals.
“When vitamin E disarms a free radical, it becomes a weak free radical itself. But unlike other free radicals, the vitamin E radical can be recycled, or turned back into an antioxidant, by vitamin C or coenzyme Q10. These network antioxidants will donate electrons to vitamin E, bringing it back to its antioxidant state.”
– Dr. Packer, author of “The Antioxidant Miracle”
Healthy cells and aerobicorganisms have more protectiveantioxidant enzymes (E.g. SOD, CAT and Glutathione peroxidase (GPX)) than anaerobic organisms – which are thus more vulnerable to oxidative damage.

Antioxidants are either enzymatic or non-enzymatic
All antioxidants need optimal amounts of certain dietary, MINERALS to function properly
(1) Enzymatic antioxidants (Biochemical enzyme catalysts, found in healthy cells of organisms)
Superoxide Dismutase (SOD). This water-soluble enzyme needsmanganese, zinc, copper (marginal to deficient in US diet), depending on location (Zinc-Copper-SOD in the cytosol, Manganese-SOD in the mitochondria.- Removes the Superoxide Radical (source of H2O2 and OH•). By catalyzing the dismutation of O2•– into oxygen and hydrogen peroxide
| O2•- + O2•- | → | O2 + H2O2 |
| Mn / Zn or Cu–SOD |
- Concentration of SOD in mammals is directly proportional to their life span!
- Found in the cells’ cytoplasm and mitochondria (energy “factories”)
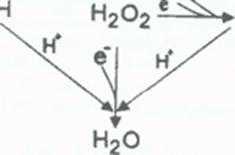
- Catalyzes decomposition of hydrogen Peroxide (H2O2) to oxygen and water
2H2O2 → O2 + 2H2O
- Highly reactive enzyme found in peroxisomes (cellular organelles containing enzymes)
- 1. Catalyze reduction of lipid hydroperoxides (ROOH) to their corresponding alcohols
| 2GSH + ROOH |
→ |
GS-SG + ROH + H2O |
| GPx |
- 2. Catalyze reduction of free hydrogen Peroxide (H2O2) (a source of hydroxyl radicals) to water. H2O2 is the preferred substrate of GPx1. Without GPx or CAT to remove hydrogen peroxide, SOD is of little value. To deal with specific problems, such as wound healing, the body produces H2O2, which must then be removed from where it is not needed, to prevent damage.
| 2GSH + H2O2 |
→ |
GS-SG + 2 H2O |
|
GPx |
(2) Non-enzymatic antioxidants
Major Water-Soluble Antioxidants
Protect aqueous areas (most of the cell’s cytoplasm) from oxidation Vitamin C (Ascorbic Acid / Ascorbate)- Nutritional requirement – since the human body cannot make it (mainly obtained from fruits and vegetables). It is largely destroyed by high heat and extended freezing (> 2 months), but dissolves in cooking water, and is thus somewhat retained in soup.
- Chelating (metal grabbing) and reducing agent (radical scavenging – donates electrons to superoxide, hydrogen peroxide, hypochlorite, hydroxyl radicals, peroxyl radicals, ozone and nitric oxide.
- Recycles vitamin E (re-reduces vitamin E radical back to vitamin E).
- Needed for the production of collagen and elastin – Involved in the formation of hydroxyproline, a major component of tissue collagen, which is the most abundant protein in our body. Collagen provides the scaffolding for, and is the main component of cartilage, ligaments, tendons and other connective tissue – the reason why broken bones regenerate and wounds heal. It is the main protein in bone and teeth. It strengthens blood vessels, preventing atherosclerosis, and along with keratin, it is responsible for skin strength and elasticity, without which we are looking at wrinkles!
- The CNS (Central Nervous System) has inbuilt double-pumps to increase vitamin C concentration. The brain and spinal cord, rich in highly unsaturated, easily oxidized fatty acids, are thus provided 100x the ascorbate antioxidant protection than in other organs.
- Involved in the synthesis of norepinephrine from DOPAMINE in the brain.
- Pro-oxidant under some circumstances
- A tripeptide (glutamyl-cysteinyl-glycine) -The cysteine provides a highly reactive free sulfhydryl group (SH) providing a “Bullseye “for oxidation by radicals. The reduced form is regenerated in a redox cycle involving glutathione reductase and the electron donor NADPH.
- Produced inside EVERY cell via glutathione peroxidase
- Helps red blood cells carry oxygen – and keep their shape, enhancing their delivery to vital organs.
- Prevents formation of methemoglobin – an altered form of hemoglobin unable to carry oxygen, produced by heme iron-oxidation.
- Aids amino acid transport across cellular membranes – needed for protein synthesis and cellular function.
- Immune system enhancer – Needed for creation / maintenance of T-Cell lymphocytes – body’s frontline defense against infection.
- Detoxifies toxins – including heavy metals, cigarette smoke, fuel exhaust, etc.
Glutathione – “King of the Antioxidants”
Major fat-soluble antioxidants
Powerful radical scavengers protect lipid membranes from oxidation Generally, these are phenols that donate a hydrogen atom to a peroxyl radical, converting it to a lipid hydroperoxide. Vitamin A / Carotenoids (E.g. β-carotene (with fat can be converted to Vitamin A), lutein, zeaxanthin and lycopene).- Detoxifies toxins – including heavy metals, cigarette smoke, fuel exhaust, etc.
- Singlet Oxygen quenchers
- Abundant in green plants, carrots, tomatoes and other brightly colored vegetables
Vitamin A – “The Grass Vitamin”
Vitamin E (Tocopherols)- Sole purpose to scavenge peroxyl radical in cell membranes
- Important in fertility
- Found in – fish oil, nuts, seeds, wheat germ.
- Present in the mitochondrion inner membrane – Important in the energy producing respiratory chain.
- Powerful reducing agent
- Found in beef, herring, chicken, trout
- Formed by sun acting on skin – or in animal products, if animal went outside in the sun, or ate foods containing vitamin D.
Vitamin D -“The Sunshine Vitamin”
Vitamin KVitamin K -“For Klotting and Kalcium”
MELATONIN- Hormone produced in the pineal gland in darkness (usually at night) – i.e. during a “Good night’s sleep”
- Direct scavenger of highly toxic OH• (hydroxyl radical),O2•– (superoxide radical), NO• (Nitric Oxide) , OONO‾(Peroxynitrite), 1O2* (singlet oxygen), and possibly ROO• (Peroxyl radical)
- May stimulate other enzymatic antioxidants – including SOD (superoxide dismutase),GPx (glutathione peroxidase), glutathione reductase, and glucose-6-phosphate dehydrogenase(G6PDH);
- Reduces oxidative damage in membrane lipids, proteins and nuclear DNA
- Enters cells with ease
- MELATONIN levels diminish with age
MELATONIN – “Darkness Hormone”
Uric Acid (most important plasma antioxidant in humans) Flavonoids Lipoic Acid Bilirubin / Histidine – singlet oxygen quenchers
"Reaction Age"
Refers to the minimum distance away a free radical scavenger must be from a radical to be able to neutralize the radical.
E.g. OH• travels no more than several Angstroms before it interacts with another molecule and a free radical scavenger must be within this “cage” to neutralize the radical. Thus, for example, if an antioxidant is confined to the lipid-rich membrane of a cell because of its specific solubility, it will be ineffective in reducing OH• damage to DNA in the nucleus.