Parkinson's Disease (PD)
Overview of Parkinson's disease (PD)
What is PD?
Parkinson’s disease (PD) is a neurodegenerative disorder that causes unintended movements of the body and behavioral changes. Neurodegenerative diseases are characterized by the progressive degeneration of neurons and share common mechanisms in the brain.
Who gets PD?
Worldwide, PD is the 2nd fastest growing age-related neurological disease after Alzheimer’s. A Parkinson’s Foundation study reports that there are currently over 1.1 million Americans and over 11 million in the world diagnosed with PD.
- Age is the highest risk factor. Incidence estimates increasing over 65 years old), although an estimated 4% of cases are diagnosed before 50.
- Men are at higher risk than women.
- Living in a high pollution area. The so-called “Rust Belt” (U.S. industrial manufacturing areas, S. California, Florida, S.E. Texas, Central Pennsylvania) have higher incidence rates. Study
- Those not consuming enough antioxidants (naturally present in organic, unprocessed fruits and vegetables). Autopsy samples of those with PD indicate increased oxidative damage of substantia nigra. PubMed PubMed Antioxidants and oxidants have a yin-yang complementary balancing effect.
Symptoms of PD
- Tremors. Usually beginning in the hand, sometimes the foot or jaw. PD tremor is characterized by a back-and-forth shaking. Predominant when hand at rest or person is stressed. A particular symptom is that the affected person rubs their thumb and forefinger together. Shaking does not usually occur during sleep.
- Rigidity. Muscle tightness resists movement
- Slowness of movement (bradykinesia). Can make simple tasks take longer
- Poor balance / postural changes. Increases risk of falling. Leaning forward whilst walking, reduced arm-swinging.
- Speech and swallowing difficulties. Forming words, speaking quietly, hesitation, slurring, talking too fast, excess saliva.
- Depression. Most common non-motor symptom of PD, estimated in ~40% of PD patients, in many cases appearing before motor symptoms. Mercury et al., 2007 Also people with depression are three times more likely to develop Parkinson’s. Tolosa et al., 2007; Ziemssen and Reichmann, 2007
- Other symptoms may develop. These include problems sleeping, oilier or drier skin, bladder / bowel / sexual dysfunction, dementia, cognitive problems (such as dementia, memory, slow thought-process), dizziness due to sudden drop of blood pressure getting up or down, fatigue, muscle pain / contractions.
- Idiopathic constipation (i.e. with unknown cause). One of the most frequent non-motor symptoms affecting up to 80% of those people with PD.
What's happening in PD?
PD occurs as a result of progressive damage or death to certain nerve cells (neurons) that produce dopamine
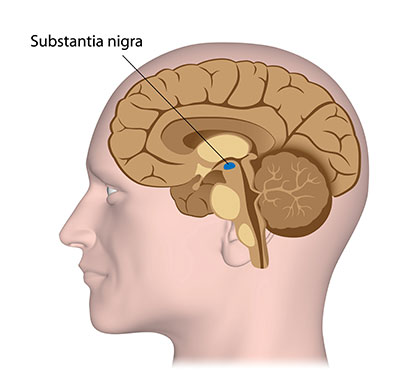
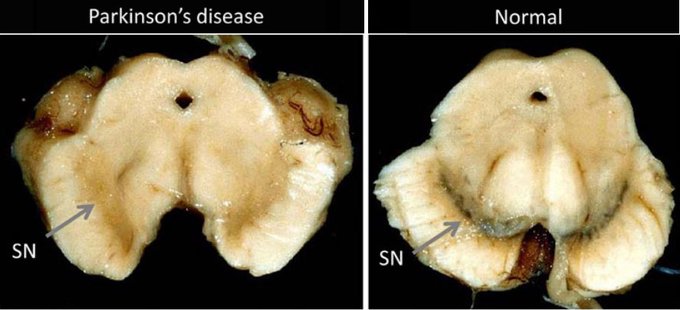
PD activity begins with damage to a crescent-shaped area of the midbrain called the substantia nigra pars compacta, a sub-division of the substantia nigra which produces most of the central nervous system (CNS) neurotransmitter dopamine.
The ventral tegmental area (VTA) adjacent to the substantia nigra pars compacta also produces dopamine in the CNS, involved in processes related to motivation, reward, and addiction, connecting to various brain regions to influence behavior and emotions. dopamine is also produced in several other locations throughout the body.
Substantia nigra is Latin for dark substance, so named for this darker colored area of the brain
The neurotransmitter dopamine (DA) is a messenger between the substantia nigra and striatum in the brain (also in other pathways in the brain and body)
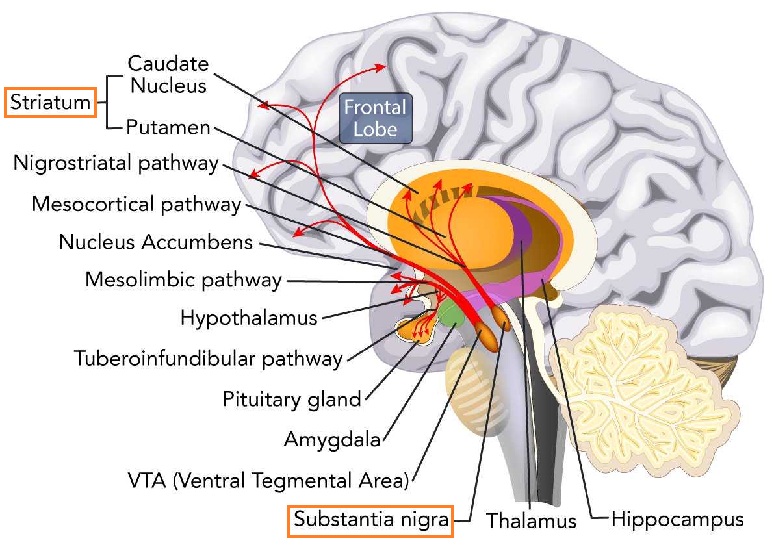
dopamine in PD
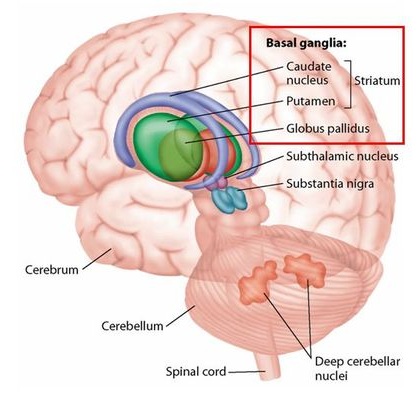
Concerning PD, dopamine is a chemical messenger that transmits signals between two functional subdivisions of the basal ganglia – the substantia nigra par compacta and the corpus striatum. The striatum, where dopamine is released, is involved with coordinating movement, decision-making, motivation and reward processing, serving as primary input to the rest of the basal ganglia, and therefore has a key role in the choices of which movement or behaviors to execute.
dopamine is used to control:
- Motor functions- visual, auditory and movement
- Non-motor functions – such as mood / emotions, behaviors, sleep, and cognitive functions, including memory.
A decrease in dopamine leads to a disruption of signals regulating these functions, with slowed movement (bradykinesia), resting tremor, rigidity and balance issues, presenting when dopamine in the brain’s striatum falls 70-80%.
dopamine production details
dopamine is a catecholamine (a neurotransmitter derived from the amino acid tyrosine). Tyrosine is obtained from high-protein dietary sources such as meat, fish, cheese, cottage cheese, milk, yogurt, peanuts, almonds, pumpkin seeds, sesame seeds, soy protein and lima beans, as well as synthesized from the amino acid phenylalanine, high amounts found in eggs, chicken, liver, beef, milk, and soybean.
In the approximately 400,000 Dopaminergic (dopamine-producing) neurons in the brain, tyrosine is converted to L-DOPA by the enzyme tyrosine hydroxylase (TH), a rate-limiting enzyme in the synthesis of dopamine. The conversion from L-DOPA to Dopamine requires the active form of vitamin B6, called Pyridoxal 5′-Phosphate (PLP), to activate the conversion enzyme L-aromatic amino acid decarboxylase (AADC).
dopamine can be converted into other catecholamines, such as the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline).
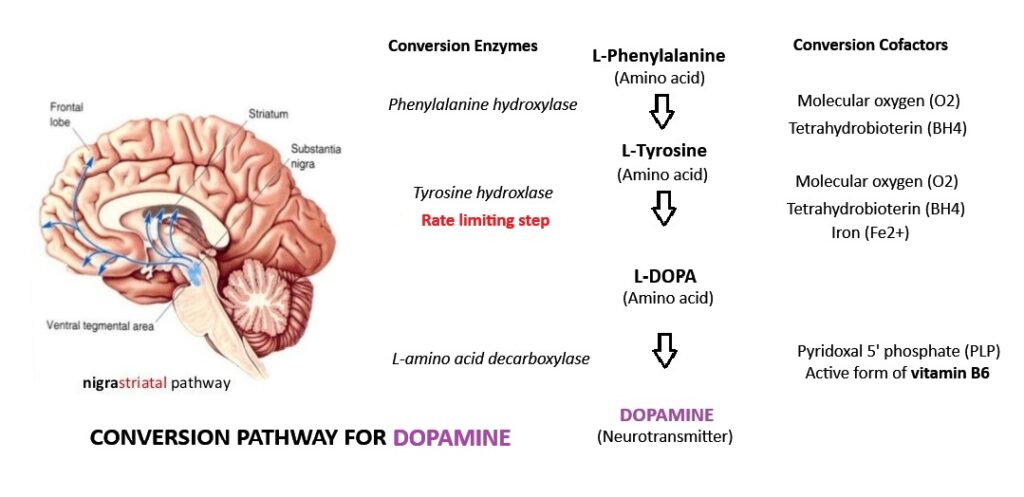
In more detail: L-Phenylalanine is converted into L-tyrosine by the enzyme phenylalanine hydroxylase, with molecular oxygen (O2) and tetrahydrobiopterin as cofactors. L-Tyrosine is converted into L-DOPA by the enzyme tyrosine hydroxylase, with tetrahydrobiopterin (BH4), O2, and iron (Fe2+) as cofactors. L-DOPA is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase (also known as DOPA decarboxylase), with pyridoxal phosphate (PLP) as the cofactor.
dopamine and LIfespan expectations with PD
Interestingly, the destruction of Dopamine-producing neurons does not directly reduce lifespan, but the consequential effects of PD-related falls or infections does impact life-length.
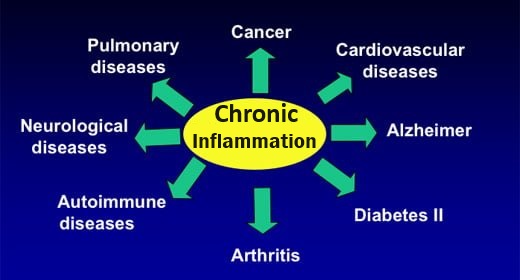
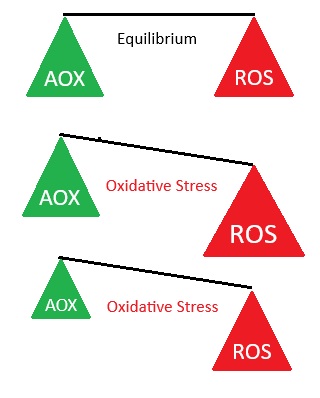
Damage to neurons occurs via oxidative stress / inflammation
This damaging process occurs due to an imbalanced presence of reactive oxygen species (ROS) compared to antioxidants. ROS target and steal the electrons of different cellular substrates, causing oxidative damage to proteins, lipids, and nucleic acids, resulting in oxidative stress and various forms of cellular dysfunction. This occurs when there are insufficient antioxidants to donate electrons to and control ROS, for example, from the normal ROS byproducts of cellular processes, or an immune response to a microbial infection or neurotoxin (e.g. cyanobacteria toxin BMAA).
Tumor Necrosis Factor-α (TNF-α)
Shown to be elevated in PD patients, TNF-α is the prototypic pro-inflammatory cytokine central to host defense and inflammatory responses. TNF’s opposing outcomes during the development of immune-mediated diseases particularly affect the CNS – effects in the CNS ranging between essential, desirable and deleterious – under certain circumstances TNF triggers cell death and tissue degeneration. Compelling evidence incriminates soluble TNF and TNFR1 (a TNF receptor) signaling in mediating the deleterious pro-inflammatory effects that promote autoimmune and neurodegenerative diseases. PubMed. Blocking soluble TNF in vivo shown to be neuroprotective PubMed
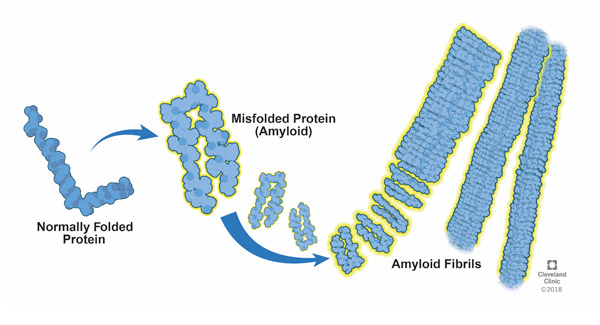
Amyloidosis - Disruptive plaque deposits disrupt cellular function and are core markers of neurodegenerative disease
Oxidative stress promotes AMYLOIDOSIS. Involved in over 50 human diseases, including PD, Alzheimer’s disease and Type II diabetes, amyloidosis occurs when abnormal (misfolded) proteins, known as amyloid fibrils, build up (aggregate) in tissue. These form plaque deposits that can disrupt normal cellular function and contribute to or lead to neuronal degeneration. Amyloid plaques block cell-to-cell signaling at synapses, a process which is essential for storing memories, processing thoughts / emotions, and planning, and are considered core pathological markers of neurodegenerative diseases.
Amyloidosis is a hallmark mechanism in PD, in which misfolded α-synuclein (α-Syn) proteins (called amyloids) form long, fibrous structures called amyloid fibrils. that aggregate into clumps (called Lewy bodies) within the neuronal cell body. A given neuron may contain one or more Lewy bodies, which occur most commonly in pigmented neurons of the brainstem, such as the substantia nigra. α-Syn modulates DNA repair processes, a function that appears to be greatly reduced in Lewy body-bearing neurons, and this reduction may trigger cell death. Amyloid buildup in the brain is linked to faster cognitive decline in individuals with PD.
α-Syn roles. Abundant in the brain, in addition to DNA repair, α-Syn has roles in synaptic vesicle recycling, neurotransmitter synthesis and release, and synaptic plasticity (brain’s ability to change / adapt to damage). The role of alpha-synuclein in neurotransmission and synaptic plasticity
- In 2024, a breakthrough study from The Neuro at McGill University revealed that Lewy bodies do not form without immune system interaction / dysfunction. Immune activation was shown to impair the vital cellular process responsible for degrading and recycling damaged proteins and organelles in dopaminergic neurons. Essentially, the neurons become overwhelmed by cellular debris, much of which is misfolded α-Syn.
Microglial (CNS immune cell) activation can damage DA neurons in multiple ways. A range of environmental factors and Lewy bodies themselves trigger microglial inflammatory responses – releasing an array of cytokines (such as TNF-α and IL-1β) and chemokines, as well as producing ROS, nitric oxide and bioactive lipids, suggesting a causal role for microglia in dopamine neuron degeneration. These microglial mediators can:
- Promote protein misfolding
- Promote the aggregation of α-Syn
- Promote BBB permeability and recruitment of peripheral immune cells
Microglial activation and neuroinflammation have frequently been detected in individuals with PD
Microglia: roles and genetic risk in Parkinson’s disease Zhang et al., 2023
The gut-brain-axis and gut microbiota
Mounting evidence suggests that the intestinal microbiota may be the triggering factor of PD pathology. Specifically, by initiating the accumulation of misfolded α-Syn in the enteric nervous system (ENS) before potential brain involvement. The ENS comprises two layers of nerve cells lining the GI tract from esophagus to rectum that communicate with the brain to control digestion, referred to as the second brain. While it can operate relatively independently of the brain, it communicates bi-diectionally with the CNS, primarily through the vagus nerve, forming the gut-brain axis, linking the emotional and cognitive centers of the brain with peripheral gut functions. Specifically, the gut microbiota has an important role in gut-brain communication affecting the neuroimmune system and maintaining brain homeostasis, thus influencing brain function and behavior. Carabotti et al., 2015
Constipation is one of the most frequent non-motor symptoms, affecting up to 80% of PD patients, and may precede the onset of motor symptoms by years.
The healthy gut microbiota can be altered by antibiotic usage or dietary changes.
Other general functions of gut microbiota include critically influencing absorption and metabolism of nutrients and toxins, development / differentiation (meaning a stem cell changes from one type to a more specialized type) of intestinal epithelium. the immune system, maintenance of tissue homeostasis and prevention of pathogenic invasion Sommer and Backhed, 2013.
Causes / Risk factors of PD
General risks
- Most cases do not run in families, but some cases may involve genetics
- Affect men more than women
- Oxidative stress. Specific “intruders” entering the brain causing oxidative damage vs. a lack of antioxidants to counter their assault. Elevated levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and decreased levels of antioxidants, such as “in-house-produced” glutathione peroxidase (GpX), superoxide dismutase (SOD) and catalase (CAT), and vitamins B6, B12, and folate (B9) have been reported in PD patients. Karahalil et al, 2022 Chia et al, 2020
- Environmental exposure to toxins
- Viral infections are a substantial risk factor and possible trigger or potential cause of PD. A common, normally considered harmless virus, human pegivirus (HPgV), brain infection was found (in post-mortem analysis) in 50% of people with Parkinson’s disease, but not in those without the condition. 2025 study. Parkinsonism was observed after the 1918 epidemic of Encephalitis lethargica (“Sleeping SIckness”), also after flaviviral encephalitis (caused by West Nile virus), St. Louis Encephalitis virus, Japanese Encephalitis B virus, and HIV (worsened on progression to AIDS and alleviated with antiretroviral therapy).
- Poor diet. A diet poor in fruit. vegetables, beans, whole grains, nuts, fish and poultry, high in saturated fat and alcohol intake increases risk for PD. According to a study of 130,000 people over 16 years. a high antioxidant intake would counter oxidative damage. The Role of Vitamins in the Course of Parkinson’s Disease
- Idiopathic constipation (i.e. with unknown cause). This critical risk factor may precede the onset of motor symptoms by years, and is associated with neurodegenerative changes in the enteric nervous system (ENS)
Drug-induced Parkinsonism (DIP) has many similar symptoms to PD and may be misdiagnosed as PD. DIP is caused by medications which block the function of dopamine (dopamine agonists), including some antipsychotic medications, dopamine depletors, SSRIs (serotonin reuptake inhibitors), and neuroleptics (a type of tranquiliser).
Mainstream treatments
Mainstream medicine offers no cure for PD
Treatments focus on:
Replacing missing Dopamine in the brain with:
- Dopamine precursors, mainly Carbidopa-Levodopa (L-dopa) which converts to Dopamine only when in the brain and unlike Dopamine, L-dopa can cross the blood brain barrier but does have unpleasant side-effects). A high protein diet reduces its effectiveness. Choose vegetables and beans over animal products. Obtaining L-DOPA directly avoids the rate-limiting conversion step from the amino acid tyrosine in an effort to boost Dopamine production.
- Dopamine agonists. Drugs that mimic Dopamine, such as apomorphine, pramipexole, ropinirole, and rotigotine.
- MAO-B inhibitors. Block breakdown of Dopamine in the brain. E.g. selegiline and rasagiline
- COMT inhibitors. Also block Dopamine breakdown, prolonging L-dopa effects.
Controlling abnormal movements using drugs. E.g. drugs that reduce production or uptake of the neurotransmitter acetylcholine. However, these treatments to not replace damaged neurons or stop progression of PD.
Nootropics. Chemical substances that improve cognitive functions, such as attention, memory, and self-control
Deep brain stimulation (DBS). Used in advance PD to improve motor function.
Alternative treatments for Parkinson's disease
Although mainstream treatments help to relieve symptoms and maintain quality of life, none can halt or slow the neurodegenerative processes. The alternative treatment approach for PD is to counter oxidative damage and maintain / regenerate neurons using research-supported modalities.
The treatment goals are:
- Treatment substances must be able to cross the blood brain barrier (BBB) and be bioavailable, or else have the ability to alter brain function via other mechanisms, such as the brain vasculature, the BBB itself or influencing the gut–brain axis by modulating gut microbiota.
- Replace the missing Dopamine
- Use treatments that will remove or destroy any toxins / microbial infection that may have gained entry into the brain causing damage to the dopamine-production area (substantia nigra pars compacta) of concern in PD. Identify and prevent continued exposure to possible toxins and/or source of microbial infection.
- Counter damaging oxidative stress (regardless of cause) with appropriate antioxidants / Reduce inflammation
- Attempt to repair neuronal damage
Examining the study results for some PD treatment antioxidants and those present or deficient in PD
Antioxidants have the potential to reduce oxidative damage in PD – these are the overall study findings: Chang et al, 2020. If possible, antioxidants should not be dissociated from their dietary sources e.g., fruits and vegetable for polyphenols, and should at least be in their natural (not synthetic form).
Some antioxidants may improve brain health indirectly—by altering cerebrovascular function, systemic inflammation, metabolism or gut‑derived metabolites—so CNS bioavailability is not necessarily a “non-starter” for benefits.
Studies having mixed results on presence of certain antioxidants in PD vs. controls
- Vitamin E. Worth mentioning, however, is that typical supplements used in studies are synthetic (dl isomer form, petroleum-based) supplements, not as bioavailable as natural d-form, plant extracts).
- CoQ10
- Vitamin D. Vitamin D in Parkinson’s disease: A systematic review of randomized controlled trials
- Creatine
- Melatonin
Studies revealing antioxidants with reduced presence in PD patients compared to controls:
- Retinoic acid and carotenoids (forms of vitamin A).
- Lycopene
- Uric acid
Polyphenols showing promise for helping PD
- Polyphenols – a major source of our dietary antioxidants. Flavonoids in particular are being most studied for their ability to improve cognitive behavior by increasing Ach presence.
Additionally –
Studies revealing substances whose presence in the body have a negative effect in PD:
- Homocysteine. Usually from eating too much protein
Omega-3 EPA and DHA
- Demonstrated to readily cross the BBB. Leclerk et al, 2021 Karalinska Institute
- Omega-3 supplementation increases CSF levels, which are bioavailable. Affects markers in the CNS for AD and inflammation. Karalinska Institute
- EPA’s anti-inflammatory properties can help mitigate the inflammatory processes that contribute to neuronal death.
- EPA has fundamental roles in neural proliferation processes. Study demonstrated EPA significantly increased neural stem cells, which generate the neurons and glia of our nervous system. Dyall et al, 2016
- EPA shown to protect neurons in a preventative role.
- Fish oil shown to counter depression, a common symptom of PD. Ticyana Moralez da Silva et al, 2008
- Supplemental omega-3 should be in a natural, triglyceride (TG) food form – not a synthetic or concentrated version. A good choice is wild salmon oil capsules
Acetylcholine (Ach) enhancers
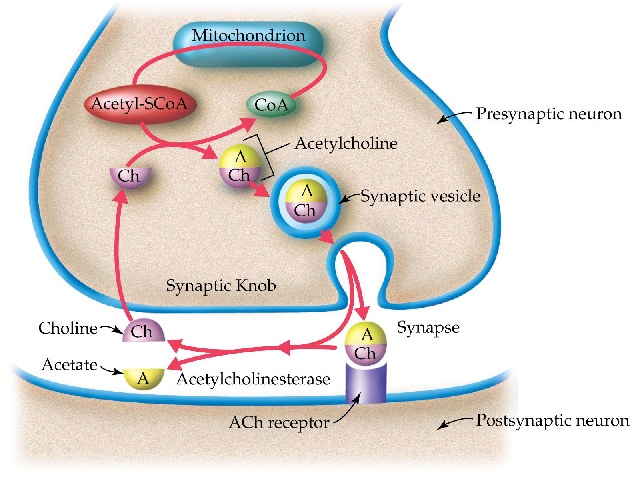
The neurotransmitter Acetylcholine (Ach) plays a major role in voluntary movement, memory, thinking and learning. Under your conscious control, Ach fires up motor neurons to stimulate muscles to contract and also has an important role in the brain’s basal forebrain nerve cells in cognitive processes. Ach itself will not pass the BBB, but its precursor choline will. This protective strategy enables the brain to produce its Ach on an as-needed basis. Once inside the CNS, choline is used by cholinergic (Ach-producing) neurons to synthesize Ach via the enzyme choline acetyltransferase.
Ach will not pass the BBB, but its precursor choline will, where it can be converted to Ach in the brain via the enzyme choline acetyltransferase. Choline-rich food sources include:
- Beef liver, Beef top round, Roasted chicken breast
- Eggs
- Cod
- Roasted soybeans, canned kidney bean
- Cooked quinoa.
- Cooked shiitake mushrooms.
- Boiled broccoli and Brussels sprouts.
- Milk (1%), nonfat vanilla yogurt
Lions Mane.This mushroom enhances activity of the enzyme choline acetyltransferase to boost Ach synthesis. Lions Mane also helps promote production of nerve growth factor (NGF) a protein that plays a crucial role in maintaining and regenerating neurons.
Acetylcholinesterase (AChE) Inhibitors Cichon et al, 2024
Acetylcholinesterase (AChE) breaks down Ach in the neuronal synapse by catalyzing the hydrolysis of Ach into choline and acetic acid. AChE inhibitors elevate Ach presence in the synapse by delaying its breakdown. Anything that can inhibit Ach break down essentially prolongs its presence in the synapse. Medicinal AChE inhibitors are designed to cross the BBB.
Dietary polyphenols, particularly flavonoids, shown to be effective AChE inhibitors, boosting Ach. Flavonoids are mainly found in skin, peel, seeds or bark:
- Quercetin – powerful neuroprotective antioxidant, reduces neuroinflammation and neuronal apoptosis (natural cell death). The most abundant dietary flavonoid, found in onions, leafy greens, apples, red grapes, berries, broccoli, citrus fruits, cherries, green tea, coffee, red wine, honey, and capers. Especially red onions (outer peel) and mango, citrus and apple peels. Choose organic or wash in water and baking soda. Crosses BBB but with low bioavailability, but there are some compounds that can improve quercetin’s effectiveness:
- Curcumin – increases permeability of BBB / enhances quercetin absorption
- Piperine – inhibits enzymes that break down quercetin, increasing its levels in the brain
- Lecithin – acts as a carrier to transport quecetin across the BBB
- Fatty acids – improve lipid solubility aiding quercetin absorption.
- α-tocopherol (form of vitamin E)
- Nanoparticles – encapsulate quercetin, enhancing its stability and transport across BBB
- Apigenin. Improves cognitive abilities. In Hypericum perforatum (buds and flowers), Grapefruit, Oranges, Parsley, Onions, Wheat sprouts, Chamomile.
- Kaempferol. Improves cognitive abilities. Protects against ß-amyloidosis. High In kale, spinach, teas, grapes.
- Eriodictyol. Strong antioxidant / anti-inflammatory /neuroprotective. Bitter-tasting flavanone extracted from Yerba santa plant (Eriodictyon californicum)
Nerve growth factor (NGF), a neurotrophin (a family of proteins that induce the survival, development, and function of neurons), is crucial for the development, survival and maintenance of basal forebrain cholinergic (i.e. Ach-producing) neurons (BFCNs). NGF robustly enhances BFCN release of Ach. Auld et al, 2001 BFCNs innervate cortical and associated structures important for attention Baxter and Chiba, 1999 and shown to degenerate in Alzheimer’s disease Bartus, 2000. Reduced NGF levels are reported in PD, and boosting glial cell-derived NGF is shown therapeutic for PD. Allen et al, 2013 However, neurotrophins do not pass the BBB and must be administered / transported by novel mechanisms.
Glutathione (GSH)
Blood and brain of PD patients show decreased GSH (reduced form of glutathione). GSH is the “King of the antioxidants” – the primary antioxidant and detoxifier in the cell cytoplasm providing a “bullseye” for oxidation by radicals.
GSH + ROS → Neutralized ROS + GS-SG (oxidized GSH)
Our defense against reactive oxygen and nitrogen species (ROS and RNS) relies on the enzymes in the glutathione antioxidant system (also the thioredoxin system), which inactivate ROS and RNS. Glutathione peroxidase detoxifies peroxides, such as hydrogen peroxide and peroxides generated during oxidation of membrane lipids. Glutathione in Brain Disorders and Aging
GSH also recycles other antioxidants, such as vitamin C and vitamin E, which have become oxidized whilst dealing with reactive species
B vitamins
- B6 (pyridoxine) – The primary cofactor vitamin in dopamine production. Its active form, Pyridoxal 5′-Phosphate (PLP), is required for the final step of the synthesis pathway, which converts the precursor molecule L-DOPA directly into Dopamine. (The conversion enzyme aromatic L-amino acid decarboxylase (AADC) is inactive unless bound to PLP).
- B6 is in fish, poultry, lean meats, starchy vegetables (e.g. potatoes), and fruits such as bananas.
Folate (B9) and Cobalamin (Vitamin B12)
- B9 and B12 are critical in the methylation cycle, one of its many aspects includes synthesis of S-adenosylmethionine (SAMe), which is a precursor for the synthesis of the raw materials needed for neurotransmitters. B9 or B12 deficiencies can potentially affect optimal Dopamine production with consequential impairment in neurological function.
- B9 is in dark leafy greens (E.g. spinach), asparagus, legumes
- Vitamin B12 is in the animal products – meat, fish, eggs, and dairy
B6, B9 and B12. Needed for production of Glutathione – King of the antioxidants
Vitamin C
Vitamin C (ascorbic acid) concentrations in the brain exceed those in blood by 10-fold or more. It readily crosses the BBB in its oxidized form – dehydroascorbic acid (DHAA), but NOT in its reduced form – ascorbic acid (AA). DHAA is reduced to AA after passing the BBB and retained in the brain tissue in the form of AA. The facilitative glucose transporter, GLUT1 (expressed on endothelial cells at the BBB), responsible for glucose entry into the brain, also transports DHAA into the brain. The mechanism of DHAA reduction to AA in the brain is not known, but believed to be related to glutathione and/or the enzymes glutathione-dependent reductases Agus et al, 1997
- Its antioxidant role can neutralize oxidants damaging nerve cells in the brain and inhibit peroxidation of membrane phospholipids.
- AA is vital for neuronal repair as well as new cell generation. Ascorbic Acid and the Brain: Rationale for the Use against Cognitive Decline
- AA boosts nerve growth factor (NGF) to aid neuronal growth
- AA supports Dopamine production since it protects Dopaminergic neurons and is a cofactor for dopamine ß-hydroxylase enzyme for production of epinephrine, necessary for the proper regulation and balance between the two neurotransmitters – norepinephrine and Dopamine.
- AA inhibits alpha-synuclein aggregation and microglial inflammation response.
Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease?
The Role of Vitamins in the Course of Parkinson’s Disease.
- The vitamin C concentration in cerebral spinal fluid (CSF) closely represents that of the brain, although is not often measured in studies. A couple of studies using mice with Alzheimer’s disease (AD) found that low C concentration in CSF and brain ECM increased oxidative stress and accelerated amyloid deposition and disease progression. Kook et al, 2014. The second study found that high vs. low C supplementation reduced amyloid deposition in the cortex and hippocampus and limited BBB impairments and mitochondrial dysfunction. Dixit et al, 2015
- Ascorbic acid (AA) sacrifices itself to recycle other antioxidants.
A dose of 1500 – 2000 mg / day is likely needed to counter the high-level of oxidative damage present in PD.
Vitamin D
Lions Mane
Lion’s mane mushroom has been used for thousands of years by Buddhist monks to increase focus during meditation:
- Helps improve cognition by enhancing activity of choline acetyltransferase, which is responsible for the synthesis of acetylcholine in the body.
- Helps promote production of nerve growth factor (NGF), a protein that plays a crucial role in maintaining and regenerating neurons.
Phytonutrients
Quercetin. (apple / mango peels). Effective AChe inhibitor (boosts ACh production)
Gingerol. (Ginger root)
Curcumin (in turmeric). Breaks down build-up of plaque (protein-clumps) on brain tissue which interrupts cell signaling. Also aids brain cell growth. A member of the ginger family (Zingiberaceae)
Drinking water
Municipal / treated drinking water is notorious for having levels of disinfection by-products at levels far exceeding health guidelines (often by 100-600 times). These compounds, including trihalomethanes (THMs, formed when chlorine reacts with organic matter), haloacetic acids, are a risk factor for PD. As such, It is advisable to drink clean water, such as Spring water with its minerals.
Alternatively, THMs are removed with a good water filter having an activated carbon component. 97% of THMS are also removed by boiling.
DMSO
DMSO has many studies supporting its healing abilities in the CNS. However, most research has been with animals; here are a few of them:
- DMSO shown to counteract neuronal damage in rats induced to have Parkinson’s. Study
- DMSO increased blood flow to brain and prevented neuronal damage / loss of memory in rats surgically modified to reduce blood to their brain (took 3 months). Study
- IV DMSO (2 weeks) counteracted chemically induced memory impairment in rats. Study.
Although DMSO is a healing modality in its own right (anti-inflammatory, antioxidant, and somewhat anti-microbial), its main attribute is its ability to penetrate cell membranes and tissues. DMSO can also combine with and ferry some healing substances with it – even through the blood brain barrier (BBB), since DMSO can open the tight junctions of the BBB and enter the CNS. This enables an anti-microbial / anti-toxin treatment substance to gain access to the brain to remove whatever is causing damage to the Dopamine-producing cells of the substantia nigra. HOWEVER, since DMSO opens a door to the brain for itself and the therapeutic substance it is carrying, it could also possibly allow passage of microbes and toxins currently in the blood. It is therefore necessary to first detox the blood using one of several methods available, The Beck Protocol and Chlorine dioxide therapy (CDT) are two very effective methods.
Other notes
Uric acid (UA)
Numerous studies have associated low serum levels of uric acid (UA) with a greater risk of developing PD, the severity of motor functions, and faster progression of both motor and non-motor features. The links are very strong and highly reproducible. One meta-analysis summarized 13 prior case-control studies of serum UA from a combined total 2379 PD cases and 2267 controls from different parts of the world. Serum UA was significantly and consistently lower in PD compared to controls. Also, serum UA was significantly lower in advanced PD compared to early PD, implying that low serum UA may contribute to disease severity or progression.
However, evidence opposes the role of UA as a biologically relevant antioxidant, and even if it were, suggests that UA is largely excluded from the brain where it could protect against neurodegeneration. It may be time to consider the possibility that something about PD causes low serum UA and not the other way around,
Uric Acid in Parkinson Disease: What is the Connection?
UA is a natural antioxidant produced during the breakdown of purines (mainly from organ meats, seafood and red meat) and can also be metabolized by drinking alcohol. Excessively high levels of uric acid are involved in gout and kidney stones.
Nicotine – lowers PD risk
A landmark 65-year follow-up of more than 30,000 British doctors published in Neurology found that smokers showed a 30-40% lower risk to develop PD than nonsmokers. Nicotine binds to nicotinic acetylcholine receptors (nAChRs) in the brain, receptors that help regulate dopamine, glutamate, and other neurotransmitters involved in motor control. These pathways influence dopamine-producing neurons. The NIC-PD trial, funded by Michael J. Fox Foundation, explored whether transdermal nicotine patches could slow progression in patients with early-stage PD. The randomized, placebo-controlled study found that did not significantly alter disease progression or symptoms. Source
Caffeine – lowers PD risk
At least six large prospective epidemiological studies have firmly established a relationship between increased caffeine consumption and decreased risk of developing PD. Animal studies have also demonstrated that caffeine confers neuroprotection against dopaminergic neurodegeneration.
Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms – PMC
References
Gao, X et al (2007) Prospective study of dietary pattern and risk of Parkinson’s disease, The American Journal of Clinical Nutrition, 86(5):1486-1494.
Barnham KJ et al (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 3:205–14.
Dyall et al, (2016) Distinctive effects of eicosapentaenoic and docosahexaenoic acids in regulating neural stem cell fate are mediated via endocannabinoid signalling pathways. Neuropharmacology, Volume 107, Pages 387-395.
Mercury M. et al (2007) The Presence of Depression and Anxiety in Parkinson’s disease Dis. Mon.
Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5:291–309.
Ticyana Moralez da Silva et al (2008) Depression in Parkinson’s disease: A double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation, Journal of Affective Disorders, Volume 111, Issues 2–3, Pages 351-359.
E. Tolosa, Y. Compta, C. Gaig (2007) The premotor phase of Parkinson’s disease, Parkinsonism & Related Disorders Volume 13, Supplement. Pages S2-S7
Wen M, Zhou B, Chen YH, et al (2017) Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS One; 12:e0173731.
Zhang Q., Cheng X., Wu W., Yang S., You H., Ye Z., et al. (2022). Age-related LRRK2 G2019S mutation impacts microglial dopaminergic Fiber refinement and synaptic pruning involved in abnormal behaviors. J. Mol. Neurosci. 72, 527–543
Ziemssen T, Reichmann H (2007) Non-motor dysfunction in Parkinson’s disease, Parkinsonism & Related Disorders. Volume 13, Issue 6 Pages 323-332