Inflammation
- Can't live with it, Can't live without it!

What is inflammation?
In simplistic terms: Molecular biology defines inflammation as a protective response, involving the activation of immune and non-immune cells in response to injured tissue with the aim to restore homeostasis.
Damage can be caused by:
- Microbial pathogens E.g. bacteria, viruses, fungi, parasites;
- Stress. Produces tissue-damaging free radicals in your body
- Diet. Consuming too much dairy / meat, too many carbs (too much sugar), damaged / altered fatty acids, and not enough healthy, anti-inflammatory fats:
- Toxins / chemicals E.g. air particulates, allergens, pesticides /herbicides, drugs etc;
- Physical injury E.g. trauma, surgery, burns, irradiation,damage inflicted by a saber-tooth tiger!
- An overzealous immune system.
The word inflammation stems from the Latin inflammare (to ignite or burn) and can be somewhat compared to a fire.
- Some fires are good – e.g. a controlled burn is confined to a specific area to clear away debris (e.g. in the body – invading microbes or damaged tissue), as in acute inflammation.
- Inflammation can burn both diseased and healthy tissues,
- There is a potential for smouldering after the fire has burned down (i.e. when the inflammatory stimuli do not stop because the causal factor(s) have not been eliminated), as in chronic inflammation
- Inflammation can spread uncontained like wildfire (e.g. throughout the body).
A main characteristic of inflammation is an increased blood flow to the tissue. Roman doctor / scientist, Aulus Cornelius Celsus (25BC-50AD), noted these 4 signs of inflammation (in latin): “tumor, dolor, rubor, and calor”, better known today as:-
- Swelling (tumor). Due to fluid accumulation as blood vessels leak fluid containing large quantities of white blood cells (leukocytes) and also serum proteins into surrounding tissues.
- Pain / Tenderness (dolor). Result from localized tissue stretching and release of inflammatory mediators that stimulate nerve endings, including release of eicosanoid lipid signaling factors (E.g. prostaglandin D2, and leukotrienes).
- Redness (rubor). A consequence of increased blood flow / vasodilation enhancing delivery of white blood cells and serum proteins to troubled location.
- Heat (calor). Also a consequence of increased blood flow.
Loss of function. Was added by Rudolf Virchow, the “father of modern pathology”, in the 19th century.
An example of inflammation is when you “catch” a cold (actually a viral infection) – your throat becomes sore, your eyes water, and your sinuses are congested. These are the physical signs of inflammation as fluid and cells build up, as a result of the immune system’s fight against a hostile invader.
Without inflammation, infections would go unchecked and wounds would never heal
Immune system (I.S.) handles inflammation in two phases: innate and adaptive
Meet the immune system participants during these two inflammatory phases
Innate immunity
1st line of non-specific immune system’s defense against “invaders” (pathogens etc). In response to tissue damage from whatever cause, first responder “sentinels” (E.g. macrophages, endothelial cells and certain activated proteins) to call in the “troops” (White blood cell (WBC) neutrophils and monocytes arising from granulocytes) either immediately or within several hours after an “invasion”. Innate immunity includes mechanical barriers, such as the skin, chemicals in the blood and immune system cells that attack invading, foreign cells.
Chemicals are released to cause inflammation attract white blood cells called phagocytes (E.g. macrophages and neutrophils) – which engulf foreign particles (E.g. chemicals, particulate matter), bacteria, parasites, dead/dying body cells and debris; being messy “eaters”, macrophages “burp up” fragments of their “meal” that alert other I.S. cells to join the fight and they also produce chemicals to kill microbes;
- Different methods are employed to kill microbes. Intracellularly, phagocyte oxygen consumption increases in a so-called “respiratory burst”, which produces anti-microbial ROS, namely: singlet oxygen, hydroxyl radical and hypochlorite; extracellularly, nitric oxide is used against bacteria; some of these oxidants leak into and damage surrounding tissue, causing more inflammation, especially if the “battle” goes on for too long;
- Tumor Necrosis Factor-α (secreted by activated macrophages) is used to destroy virally infected cells / cancer cells:
- Pus – is a combination of dead tissue, dead bacteria, and live and dead phagocytes.
The damaged / infected site becomes red, swollen and warm, with possible loss of function – as healing mechanisms are initiated.
Eventually any damage is repaired – fibroblasts manufacture the extracellular matrix (ECM) and collagen to repair damaged connective tissue (admittedly sometimes leaving a scar);
Acute inflammation response is designed to work best in a well-nourished person with a mild to moderate injury / infection over a short period of time – response usually occurs within minutes to hours, and the problem is often fixed in a few days;
Adaptive / aquired immunity
Definition of antigen: A pathogen that should not be in the body, such as a toxin, a diseased cell, or pathogenic bacteria, virus, fungus or parasite
- 2nd line of systemic defense to protect against re-occurence of a specific antigen in the body – Adaptive immunity takes some time to provide “Specialized Troops” to recognize and remember specific antigens to be ready to protect against their re-infection or recurrence.
- HUMORAL immunity – involves production of specific antibodies (ABs) in response to a specific antigen and is mediated by B-lymphocytes. ABs recognize the difference between “friend and foe” and act as flags or markers for specific antigens. ABs patrol the humors, i.e. the blood and lymph fluids between cells to combat antigens (e.g. ABs can attach to specific microbial pathogens that have been in the body at an earlier time, to flag them for destruction before it starts multiplying).
- CELL-MEDIATED immunity – involves production of cytotoxic T-lymphocytes, activated macrophages, activated NK cells, and cytokines in response to an antigen and is mediated by T-lymphocytes.
- Macrophages call in the lymphocytes (B-cells and T-cells) – these are mobilized to find the non-self invader (antigen):
T-cells – identify and eliminate antigens.
B-cells – produce specific antibodies (think of them as flags, at least 10,000 of them protruding from their surface cellular membrane) to attach to a specific antigen to “tag” it for destruction by cytotoxic T-cells (Natural killer T-cells / NKT). Note, these are distinct from Natural Killer (NK) cells of innate immunity;
Helper T-cells – signal B-cells to multiply to strengthen attack force;
Suppressor T-cells – down-regulate other I.S. responses;
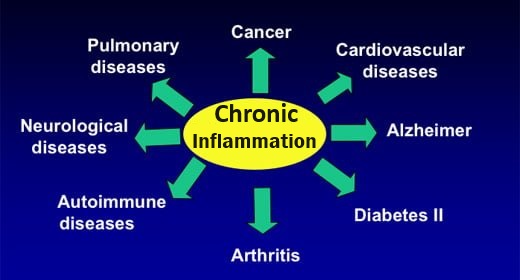
Inflammation is categorized as either ACUTE or CHRONIC
ACUTE (short-term) inflammation. The result of an immediate, necessary and appropriate response to injury or infection.
CHRONIC low-level inflammation (CLLI) is a common factor in most health problems. This response occurs if an infection / injury continues when the inflammatory response is unequal to the task of resolving the problem. Unresolved inflammation causes ongoing tissue damage, sometimes felt as pain. Others go unnoticed until you are diagnosed with one of the diseases of our time – including heart disease, cancer, diabetes, asthma, inflammatory bowel disease (IBS), auto-immune disorders such as rheumatoid arthritis and, multiple sclerosis, When the inflammation response is over-exaggerated, the body can face problems such as sepsis, sepsis shock and severe reaction to COVID-19.
Meet the immune system participants in the inflammatory process
| Immune System | I.S. | |
| White Blood Cells (aka leukocytes) | WBCs | Provide action against infection or injury |
| Extra-Cellular Matrix | ECM | Network of connective tissue, which fills spaces between cells, provides strength, binds cells and tissues together and links almost every cell in the body; |
| Cell surface Adhesion Molecule | CAM | Enables a cell to adhere to other cells or molecules in the ECM; specifically promotes adherence of WBCs (e.g. monocytes) to endothelium; includes Intercellular CAM-1 (ICAM-1), Vascular CAM-1 (VCAM-1), E-Selectin |
| Fibroblasts | Connective tissue cells that secrete precursors of all ECM components. | |
| Oxidative stress | A situation where reactive oxidants (i.e. free radicals) reactive oxygen species (ROS) and reactive nitrogen species (RNS) can cause damage to cells if there are insufficient antioxidants to control them. | |
| Antigen (antibody generator) | Ag | Our body recognizes an antigen as a foreign substance – such as pathogenic microbes, toxins from snake or insect bites and allergens (e,g, pollen) and other harmful particles. Our innate and adaptive immune systems promote the formation of an antigen-specific antibody. An antigen can bond to (i) an antibody antigen-binding site or (ii) a T-cell antigen receptor. Each T-cell antigen receptor has a specificity for a single antigen, in order to recruit only the lymphocytes required for that antigen. Antibody antigen-binding sites have the ability to “cross-react” to bind more than one antigen, Antigens exist on normal cells, cancer cells and/or microbes |
| Antibodies (immunoglobulins) | Ab | 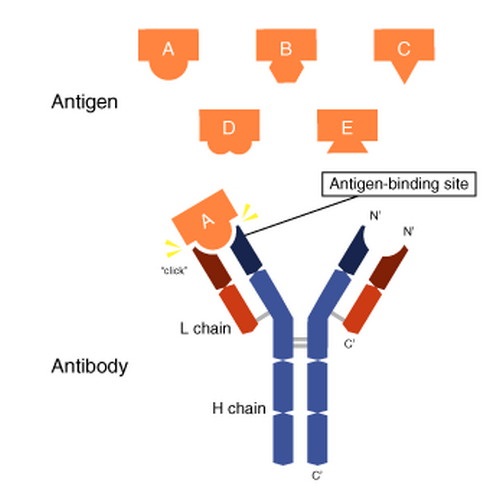 Used by the I.S. to identify and “tag” an antigen (particles, toxins, infected, damaged or cancerous cells) for removal by other parts of the I.S. Produced by B-cells, Abs are Y-shaped proteins belonging to the immunoglobulin family (IgA, IgD, IgE, IgG, IgM). The I.S. inserts antigen-specific Abs into the membranes of B-cells (specific to the same antigen). When armed with receptors (antigen-binding sites) at the tips of the Y of the Ab, they migrate to lymph nodes or the spleen. An antigen matching the Ab receptor, causes the B-cell to form the Mh2 complex, which it inserts into its plasma membrane. T-helper cells recognize the antigen Mh2 complex, which stimulates B-cell proliferation and differentiation into:
IgA – in tears, saliva, mucus, breast milk, intestinal fluid – protects against inhaled and ingested pathogens (organisms / microbes that can cause disease) |
| Endothelial cells | ECs | Endothelial cells form the endothelium – a single layer of squamous cells lining the interior surface of blood and lymphatic vessels, an interface between blood or lymph and the rest of the vessel wall. |
| Phagocytes | WBCs that engulf / ingest and destroy invading micro-organisms, cellular debris, and dead cells
Activated Neutrophils and macrophages produce reactive oxygen species (free radicals) – referred to as a respiratory burst. ROS chemically react with other substances to become potent microbicidal substances to add to the I. S. arsenal. | |
| Myelo-peroxidase | MPO | A lysosome (cell organelle that digests cellular waste inside and outside cell), most abundantly secreted from neutrophils, with oxidative stress being a factor. It proces hypochlorous acid from hydrogen peroxide. Contains heme (the green pigment seen in mucus and sputum). |
| Cytokines |
| |
| Granulocyte cells | Part of innate I.S. (1st line defense) characterized by so-called ‘specific granules’ in their cytoplasm (all material enclosed by cell membrane, except cell nucleus). Cell-killing molecules are released from granules when activated by an immune stimulus, in a process called degranulation.
| |
| Platelets | Modulate clot formation and have roles in inflammation, infection, immune response and cancer. Gather at damage site, adhere to WBCs, Subsequently release cytokines / chemokines, which are chemotactic for (i.e. mobilize) neutrophils and monocytes. Target lymphocytes, neutrophils and monocytes to inflammation sites, triggering further inflammation. Activated platelets release SEROTONIN (by degranulation). Causes itching ,and increases vasodilation vessel permeability. (SEROTONIN relates to muscular tone (TONIN) in blood (SERO) vessels) Platelets also engulf microbes. | |
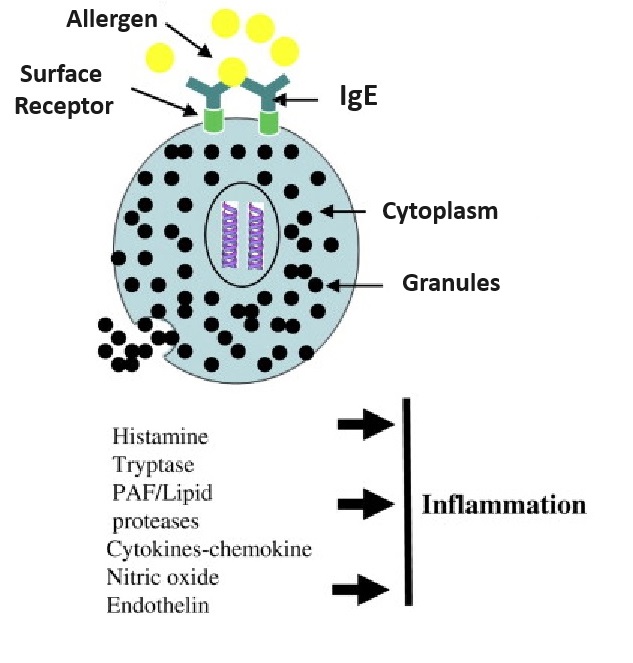
| Mast cells | MCs | Mast cells are your body’s alarm system – Key activists initiating the inflammatory process. These WBC granulocytes (made in bone marrow) are resident in connective and mucosal tissues (line membranes in body cavities, and cover organ surfaces). MCs are mostly present in and act upon tissues surrounding blood and lymphatic vessels, nerves, skin, urinary tract, mouth, conjunctiva, nose and numbers are elevated in and mucosa of asthmatic lungs, and GI tract of inflammatory bowel disease (IBS). MCs don’t destroy pathogenic invaders themselves, but “sound the alarm” for mediators to deal with the problem, creating inflammation, and then return the body to normal. Sometimes the immune system reaction can be over-zealous, mis-identifying a particle as a toxin, leading to such as seasonal allergies or allergic asthma. Mast cells are coated with a variety of receptors and quickly “sound the alarm” to protect tissue in their “neighborhood” against damage. They begin the inflammation process (in both the innate and adaptive immune response) when surface receptors are activated by physical injury or antigens, such as allergens, pathogenic microbes, harmful particles, toxins, venoms (e.g. from snake or insect bites), proteases, complement proteins, or chemical substances. When engaged by the appropriate ligand (E.g. LPS / Endotoxin of gram-negative bacteria, peptodoglycan of GRAM-POSITIVE BACTERIA), it triggers release of granules (by exocytosis), some discharging their inflammatory mediators immediately, others later.Mast cells are known for their role in allergies and anaphylaxis, but also have protective roles in wound healing (causes blood vessels to dilate allowing more blood and immune cells to reach damaged area), angiogenesis, pathogenic defense +++.Stimulus-specific mast cell mediators are released into the extracellular “neighborhood” – initiated by antigen or injury-activated MC surface receptors – these mediators either initiate inflammation or deal with the damage: |
- Released via mast cell degranulation:
- Histamine, in response to injury or toxic substance; increases vessel dilation / permeability, promoting blood flow to area and leakage of fluid, pathogen-fighting WBCs and proteins from blood into the tissue space. This causes redness, warmth, swelling, itching and pain.
- Heparin (an anticoagulant),
- Serine proteases (e.g. trytase, chymase),
- Myeloperoxidase enzyme (MPO) – most abundantly by neutrophils. Can oxidize LDL in the space just below the endothelium.
- Mast cells synthesize (de novo):
- Eicosanoids (lipid signaling factors) from membrane omega-6 arachidonic acid. Consuming a balance of both inflammatory (most omega-6 fats) and anti-inflammatory omega-3 EPA and DHA, and omega-6 GLA) fatty acids will ensure a balanced response when dealing with injured tissue; EFAs ==> Local Hormones – First Response Team
- Prostaglandin D2 (PGD2) – bronchoconstricting, pivotal roles in antigen presentation, leukocyte activation, ECM deposition, fibrosis.
- Leukotriene B4 (LTB4) – chemoattractant for neutrophils
- Leukotriene C4 (LTC4)
- Chemoattractants (E.g. chemokines, cytokines) – which attract leukocytes (white blood cells) to the “scene of the crime”. Large amounts of TNF-α released by stimulated mast cells as a result of pathogen exposure.
- Chondroitin sulfate
- Mast cells (and basophils) cause formation of bradykinin (BK) (by releasing heparin)
- The bradykinin receptor B1 is expressed only as a result of tissue injury. BK (produced from an inactive precursor always circulating in the blood) is released from mast cells and basophils,
- Dilates blood vessels / lowers blood pressure (by stimulating NITRIC OXIDE and increases blood vessel permeability. To allow needed blood components to enter the tissue space; ACE inhibitor drugs reduce blood pressure by increasing bradykinin.
- Contracts airway smooth muscle and is a potent bronchial vasodilator, stimulates mucus secretion and coughing. BK activates release of neuropeptides from sensory nerves in airways, leading to reflex bronchoconstriction, coughing and neurogenic inflammation. Fuller RW, Dixon CM, Cuss FM, Barnes PJ. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am Rev Respir Dis. 1987 Jan;135(1):176-80. PubMed
- Effects produce redness, warmth and swelling and is Involved in pain mechanism
- Stimulates phospholipase. Increases the production of prostaglandins (local “hormones”)
These mediators recruit all types of white blood cells to the site, many of which are activated to produce their own inflammatory mediators. Types of white blood cells recruited include:
- Monocytes. Become macrophages (“big eaters”) when they leave the blood and enter the tissue
- Neutrophils. Squeeze through capillary walls and into infected tissue to kill invaders and then engulf the remnants by phagocytosis.
- Antigen-presenting dendritic cells. Main function is to process antigen material and present it on the cell surface to the immune system’s T-cells, thereby acting as messengers between the innate and the adaptive immune systems).
- All kinds of lymphocytes:
- Natural Killer (NK) cells. Specialized to kill certain types of target cells (esp. virally infected or cancerous host cells) in innate (1st line of defense) immunity
- B-cells and T-cells. These cells lead to an adaptive immune response (2nd line of defense which “remembers” that the problem has happened before and is prepared for it);
- Eosinophils. Blood levels increase sharply with parasitic worm infections. Eosinophils release the cytoxic contents of their granules on the “invader”.
Matrix metallo-peptidase-9MMP-9MMP-9 is one of the matrix metalloproteinase enzymes that degrade ECM proteins and can degrade the fibrous cap on plaque. MMP-9 digests ECM decorin, elastin, fibrillin, laminin, gelatin (denatured collagen), and collagen types IV, V, XI and XVI; MMP-9 levels are elevated during inflammation in CHD, arthritis, pulmonary-emphysema, diabetic retinopathy;Lymphocytes
Small WBCs of the ADAPTIVE I.S.
- T-cells – “Killer” (cytotoxic) T-cells directly kill infected, damaged, or cancer cells presenting antibodies on cell surface used to “tag” problem cells; “Helper” T-cells – release cytokines, further activating killer T-cells and memory B=cells
- B-cells. Make antibodies in response to antigens and abnormal cells (e.g. cancer cells). Two types:
- Plasma cells: produce / release Abs in response to antigens
- Memory cells: remember particular antigens for future defense if pathogen returns
Large granular WBCs of the INNATE and ADAPTIVE I.S.
- NK-cells (natural killer cells) – similar to “Killer” T-cells, quickly responds to and kills infected, damaged, cancer cells, especially highly proliferative cells, Unlike “killer” T-cells which need a cells to be “tagged”, NK-cells recognizes them via cell-mediated immunity ( i.e. without detecting antibody presence), based on activating and inhibitory receptors, and kills pathogens by secreting cytotoxic granules
Acute phase proteins (APPs)
In response to infection or trauma, APPS are expressed by most cells – including activated endothelial cells (ECs), mast cells and macrophages, and used in cellular communication to modulate cell growth, development, repair, fibrosis, inflammation, immunity and other processes to maintain body in a steady state; Blood plasma concentrations of specific APPs, called acute-phase reactants (APRs), will either increase or decrease in response to inflammation.
ACUTE-PHASE REACTANTS (APRS)
Most produced by liver (some by other cell types, including monocytes, ECs, fibroblasts and adipocytes)
SOME APRS INCREASE DURING INFLAMMATION (CALLED POSITIVE ACUTE-PHASE APRS)
For example:
- C-reactive protein (CRP) – not normally present in blood, a reliable risk-marker of inflammation / atherosclerosis.
- Activates the complement system (immune system defense mechanism) to opsonize (add a negatively charged coating to) microbes in preparation for their destruction.
- Enhances phagocytosis of low-density lipoprotein, leading to the formation of foam cells;
- Data suggests CRP may increase CAM expression.
- Complement factors – complex series of protein molecules circulate in the plasma and other extracellular fluids in an inactive form. Enzymes convert complements into biologically active molecules when exposed to microorganisms or other foreign substances to destroy or mark cells for destruction
- Coagulation factors. Fibrinogen, prothrombin, plasminogen.
OTHER APRS DECREASE DURING INFLAMMATION (CALLED NEGATIVE ACUTE-PHASE APRS)
- Albumin
- Transferrin
- Anti-thrombin – decreasing it allows blood to clot
- Retinol-binding proteins.