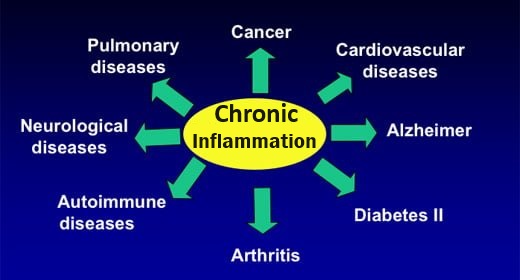
Causes of chronic low-level inflammation (CLLI)

CLLI is a common factor in the progression and development of most health problems / diseases today primarily due to dietary and lifestyle changes:
SOME OF THE CAUSES | DETAILS |
|---|---|
| Oxidant / Antioxidant Imbalance | Oxidant-promoting stress, toxins, damaged fats, microbes and NOT enough countering antioxidants |
| NOT having enough anti-Inflammatory fats to counter inflammatory fats | Damaged fats from typical grocery / restaurant oils. An insufficiency of anti-inflammatory omega-6 GLA and omega-3 EPA and DHA.to oppose inflammatory omega-6 fats. Lack of healthy SATURATED fats. Nutrient deficiencies |
| Too much sugar / HFCS and other refined carbohydrates | Hyperglycemia / Glycation produces damaging advanced glycation endproducts (AGES) |
| Too much meat & dairy and NOT enough B-vitamins | Elevated homocysteine levels |
| Overly acid-forming diet / dehydration | NOT enough alkaline-forming foods / dehydation |
| Lack of sleep and emotional stability | |
| Food contaminated with pesticides and herbicides or genetically modified; chemically treated water; excessive exposure to environmental chemicals and drugs; |
Inflammation occurs as a result of oxidant / antioxidant Imbalance - caused by stress, toxins, damaged fats, microbes and NOT enough antioxidants
A major reason damage occurs to body tissue is because too many highly reactive oxidant ions / molecules in the body have overwhelmed the body’s antioxidant presence – Uncontrolled free radicals: ROS (Reactive Oxygen Species), RNS (Reactive Nitrogen Species) and other reactive species are likely to take part in chemical reactions with your body’s proteins, lipids, carbohydrates and DNA. Oxidants are either introduced into or produced in the body for necessary functions (e.g. metabolism, immune activities).
For information on dual roles of Reactive Oxygen Species (ROS) activities in the body:
Life’s Oxygen Paradox – Meet Dr. ROS Jeckyll and Mr. ROS Hyde
ROS in the body come from various sources – some are mentioned below, but for more detailed information:
Where do ROS in the body come from?
Reactive oxidants are produced in the body during metabolism
Energy-producing oxidative metabolism produces ROS – Oxidative metabolism is part of the design to extract energy by controlled oxidation of substrates, at the same time preventing uncontrolled oxidative damage via antioxidants – a good analogy is “To use fire for warmth, but to not get burnt”.
ROS in oxidized lipids / trans fats
- Oxidized cholesterol
- In fried, cooked, cured, aged, or processed foods, chiefly meats, eggs and dairy – E.g. powdered eggs/milk, scrambled eggs. Dietary oxidized cholesterol is equally distributed to both HDL and LDL in the body. (Univ of Ca, 2003)
- Cholesterol produced by the body or consumed in food is oxidized in the body – in its antioxidant role when it comes into contact with free radicals. (lipid peroxidation induced by ROS / RNS seems to be involved not only in cardiovascular disease, but also in cancer, rheumatoid arthritis, and other degenerative health problems, including accelerated aging).
- Oxidized polyunsaturated, omega-6 and omega-3 fats. These essential fats are easily oxidized by ROS and RNS:
- In food before consumption. E.g. during the usual high-temperature commercial process of extracting vegetable oils from seeds, or in high-temperature processed foods. E.g. fried foods. Dietary omega-3andomega-6fats are essential to well-being, but need to be consumed undamaged, in balance, together with fat-protective antioxidants, such as vitamins A, D, E. and K.
- In the body after consumption – when antioxidants are deficient; particularly damaging to cell membranes.
Trans Fats
- Follow-up to Nurse’s Health Studies – found that those consuming the most trans fats had a significantly higher risk of cardiovascular disease. Yu E et al, 2016
- Increase both LDL and Lp(a) – One study showed significant increases in Lp(a) levels of subjects consuming diets high in trans fats, but not in those consuming high levels of saturated fats J Lipid Res 1992 Oct;33(10):1493-501. Nutritionist/author Dr. Mary Enig maintains that saturated fats actually LOWER Lp(a) levels. (Lp(a) is a specific type of LDL cholesterol, implicated in, and an accurate marker for CVD)
- Decrease HDL
ROS produced by white blood cells when body reacts to an adverse factor
- A wound, fever, nervous imbalance (stress), microbial infection or toxin precipitates an inflammatory response – in which ROS, RNS or other reactive oxidants are released by immune system white blood cells (E.g. macrophages).
Emotional stress (Possibly today’s main oxidation-causing stressor)
- Histamine is produced as a result of erratic stress. Accumulating histamine leads to inflammation and plaque formation. (Bruce H. Lipton’s histamine theory is that erratic stress induces mast cells on blood vessel endothelium to emit histamine, which causes cells to multiply).
- Having a type-A personality is linked to an increased risk of CHD. Characteristics include time urgency and competitiveness.
Trauma / Infective microbes
- Physical damage or presence of infectious agents, such as bacteria, viruses, protozoa, initiates an inflammatory process that leads to ROS production by phagocytes – E.g. infectious bacteria Chlamydia pneumoniae and the Herpes simplex virus have been proposed as initial inflammatory infectious agents in atherosclerosis.
- ROS are released in the synthesis of prostaglandins and leukotrienes. Local “messenger” molecules produced / released from omega-6 arachidonic fatty acids are produced in response to trauma.
Environmental toxins
- Induce inflammatory response leading to damaging ROS and RNS – E.g. cigarette smoking by-products, exhaust fumes, household chemicals, chlorine, fluoride, heavy metals, pesticides/herbicides, GMOs, food additives: E.g. dyes, aspartame, sucralose, MSG, nitrites (used to cure meat such as bacon, bologna, corned beef, sausages and hot dogs); Suwa et al, 2002; Pope et al, 2004a
ROS produced by hyperglycemia (chronically high blood sugar levels)
- Hyperglycemia induces an inflammatory reaction in endothelial cells (lining interior surface of blood vessels), which can cause an increase in the production of ROS (reactive oxidants, including free radicals) Ceriello et al, 1996
- Hyperglycemia increases the formation of oxidized LDL – an important modulating factor in atherosclerosis and cardiovascular death.
NOT having enough anti-Inflammatory fats to counter inflammatory fats causes inflammation
The type and quantity of essential fats you eat has an effect on the body’s inflammatory response
- Certain essential fatty acids located in cell membranes produce specific “hormone-like” substances (called eicosanoids) in a cell’s immediate environment, to effect either an inflammatory or anti-inflammatory action. The most well-known of these eicosanoids are prostaglandins, leukotrienes, thromboxanes and prostacyclins.
- Typically the problem is not having enough anti-inflammatory omega-6 GLA and omega-3 EPA and DHA. Omega-6 fats yield both inflammatory and anti-inflammatory eicosanoids; Omega-3 fats have only an anti-inflammatory effect;
Fats that work towards reducing inflammation
- The essential omega-3 fatty acid EPA in cell membranes is converted by the COX and LOX enzymes to anti-inflammatory prostaglandins and leukotrienes. Omega-3 EPA is obtained from our diet in such as:
- Marine foods and supplements contain EPA. Including oily fish (E.g. salmon, herring, trout, mackerel), fish oil (E.g. Wild salmon oil) and krill oil; Antarctic Krill Oil Eating fish for omega-3 EPA /DHA
- Plant foods flax, hemp and chia seeds, and also walnuts are rich in alpha-linolenic acid (ALA) that can be converted to EPA, to yield anti-inflammatory eicosanoids via the COX and LOX enzymes – however the conversion process requires certain enzymes, which themselves need specific vitamins and minerals, which may be deficient in certain people, such as the elderly, certain races, and those who are ill;
Can we convert plant O3 ALA to the needed EPA / DHA?
Flax Seed, Chia Seed, Hemp Seed
- Omega-6 fats that yield anti-inflammatory Series-1 prostaglandins (PGE-1) from dihomo-gamma linolenic acid (DGLA)
- Omega-6 linoleic acid (LA) found in nuts and seeds, but more often in typical grocery store oils (E.g. corn, soybean oil) and processed foods containing them – can be converted to GLA and then converted via enzymes to DGLA to yield anti-inflammatory eicosanoids; DGLA can also be derived from GLA found in high amounts in borage, evening primrose and blackcurrant oils, which are the usual supplement sources for DGLA (which is only found in mother’s milk in any quantity); PGE-1 molecules are about 1/2 of your inflammation-fighting capability. Without them, you have a persistent tendency toward inflammation, which often leads to INSULIN resistance, obesity and many deadly diseases.
Fats that tend to increase inflammation
- Omega-6 Arachidonic acid (AA) essential fatty acids yield inflammatory eicosanoids.. Found in meat and dairy yields inflammatory prostaglandins and leukotrienes; Linoleic acid (LA) fatty acids found in typical grocery store oils and processed foods can be converted to AA;
For more information on the eicosanoids, how to optimize conversion enzymes and how to ensure a healthy intake of the essential fats:
Hyperglycemia – Too much sugar / HFCS and other refined carbs causes inflammation
Hyperglycemia /Glycation increases damaging AGEs
Why the hyperglycemia?
- Excess dietary sugars and refined carbohydrates increases blood glucose – usually accompanied by prolonged high insulin levels.
- Excess consumption of high fructose corn syrup (HFCS) – in processed foods, sodas etc;
- INSULIN resistance as in Type 2 diabetes – further increases blood sugar levels. Organs and tissues NOT dependent on INSULIN for their absorption of glucose are more susceptible to damage from periods of hyperglycemia than other organs – i.e. kidneys, blood vessels, peripheral nerves and lenses of the eye.
- Hyperglycemia is positively correlated with arterial disease in many diverse populations
Glycation has inflammatory effects
Glycation is the covalent attachment of a protein, lipid or nucleic acid molecule to a sugar molecule without enzymatic control.
- AGES
- AGES are proteins or lipids that have become glycated to sugar molecules to form a glycoprotein or a glycolipid. Advanced glycation endproducts, aptly called AGES, are a bio-marker implicated in age-related chronic diseases including degenerative diseases, such as diabetes mellitus, CVD, chronic kidney disease and Alzheimer’s disease. Fructose and galactose have about 10 TIMES the glycation activity of glucose. Fructose has become excessively present in today’s Western diet due to the use of High fructose corn syrup (HFCS) in many processed foods (Bunn & Higgins, 1991)
- AGES can be formed exogenously – AGEs are typically formed when sugars are cooked with proteins or fats. Also, food manufacturers add AGEs to foods as flavor enhancers and colorants to improve appearance;
- AGES can be formed endogenously – glycations occur mainly in the bloodstream to some of the absorbed simple sugars:glucose, fructose, galactose. AGEs can directly damage biomolecules, such as the endothelium, connective tissue collagen, elastin and fibrinogen (E.g. in blood vessel walls).
- Circulating AGEs bind LDL cholesterol and increase its accumulation in the arterial wall – glycation of LDL cholesterol increases the proportion of lipoproteins that are taken up via inflammatory cells and decreases the proportion taken up by liver cells (hepatocytes) via classical LDL receptors, thus contributing to atherosclerosis by increasing accumulation of LDL cholesterol in the artery wall.
- Scavenger cells with special receptors for AGEs, called RAGEs – again how aptly named is that!? – these RAGEs “get mad” and bind to the AGEs to remove them.
- Glucose and fructose can glycate and deactivate the enzyme glutathione reductase. This enzyme is responsible for maintaining the body’s major cell-protecting antioxidant enzyme Glutathione (GSH). Glucose, glucose 6-phosphate and fructose all displayed a time-dependent inhibition of glutathione reductase activity, suggesting that these sugars glycate this enzyme. (Blakytny et al, 1992)
- Increased amount and duration of glucose in the blood allows more glycation to occur, inflicting damage on tissues via lipid oxidation. (Bucal, 1993);
Too much meat and dairy and NOT enough B vitamins
Elevated amounts of homocysteine (Hcy) produced from the amino acid methionine in meat and dairy can cause inflammatory damage in several age-related diseases. Hyperhomocysteinemia is shown in osteoporosis, Alzheimer’s disease, Parkinson’s disease, and cardiovascular disease (CVD), and associated with (but not limited to) cancer, aortic aneurism, hypothyroidism, end renal stage disease Damage is caused by inflammatory cytokines to such as collagen and elastin in connective tissue; Kumar et al, 2017- Homocysteine (Hcy) is not DIRECTLY obtained from the diet. Hcy is formed from the metabolism of the essential amino acid, methionine, abundant in red meat and dairy products. Vegetables, with few exceptions (e.g, sesame seeds and Brazil nuts), are low in methionine;
- Adequate amounts of folic acid (B9) and other B vitamins are needed to convert homocysteine into less toxic amino-acids – Vitamin B2 (riboflavin) and magnesium are also involved in homocysteine metabolism.
- Common levels in Western populations are 10 – 12 μmol/L. Levels of 20 μmol/L are found in populations with low B-vitamin intakes or in the older elderly.
- Higher Hcy levels are associated with various health problems. Including cancer, diabetes, CVD, age-related macular degeneration, hearing loss, brain atrophy, dementia, migraine and thyroid disease, prescription drug use, age, declining ability to absorb B12, deteriorating kidney function, smoking, coffee consumption, and excessive alcohol intake, lack of exercise, obesity, stress, and inheriting a variant gene for MTHFR;
- Homocysteine levels can be reduced by:
- Remethylation (~50%) – utilizes active folate (MTHR), B12 and the enzyme MTHFR to convert homocysteine back to methionine. (Also this conversion occurs in kidney and liver via betaine homocysteine methyltransferase (BHMT) which transfers a methyl group to homocysteine via the demethylation of trimethylglycine (TMG / aka betaine, which serves as a methyl donor) to dimethylglycine (DMG)) ;
- Transsulfuration (~50%) – utilizes the active form of vitamin B6 (pyridoxal-5′-phosphate) and the enzyme cystathionine-synthase (CBS). Once formed from cystathionine, cysteine can then be used by the body to make protein and glutathione (GSH), a powerful antioxidant.
If either of these pathways are impaired (E.g. due to a deficiency of B6, B12, folate, betaine), then plasma fasting Hcy concentrations are increased, significantly so in the remethylation pathway.
- Homocysteine (Hcy) levels can be increased by:
- Low thyroid activity – which depletes intracellular magnesium promoting Hcy accumulation. McCully (1983).
- Fluoride, Chlorine, and bromine. These goitrogens lower thyroid activity and thus increase Hcy. Iodine supplementation counters these goitrogens; Yiamouiannis, J.(1986); McCully. K.S. (1983)
- The rare hereditary disease homocystinuria (HCU) elevates Hcy levels. An inherited autosomal recessive trait (both parents carry defective gene) of a disorder of methione metabolism. This can be caused by a deficiency of the enzyme cystathionine-synthase (CBS) or methione synthase, or B6, B9 or B12 deficiency, or those people with a genetic trait, which decreases activity of an enzyme called MTHFR (see inset). MTHFR metabolizes folic acid to its active form; however, a significant reduction in plasma Hcy is achieved by taking an active folate (5-MTHF) supplement.
MTHFRAn enzyme used to produce a substrate involved in the beneficial conversion of Hcy to methionine. A DNA sequence variant (polymorphism) in MTHFR results in its decreased enzymatic activity. This trait is present in about 10% of the world population and it is linked to an increased incidence of thrombosis and CVD, occurring more often in people with above minimal levels of Hcy (~6 μmol/L). |
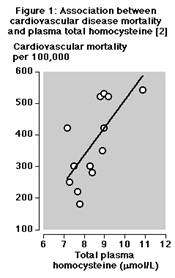
- Studies indicate that those with Hcy values >or = 6.3 µmol/L are at increased risk of atherosclerosis.
- Women have 10-15% less Hcy during their reproductive decades than men which may help explain the fact they suffer heart attacks on average 10 to 15 years later than men; A practical Hcy goal after age 50 is < 7-8 μmol/L;
- Hcy is associated with higher risk of strokes. Rabelo, 2022
- Hcy is a ‘corrosive’ of the three main structural components of the artery. i.e. the connective tissue proteins collagen, elastin and proteoglycans (e.g. fibrillin is a glycoprotein needed to form elastic fibers). Hcy prevents the formation of stable peptide bonds by tending to cleave to itself.
Credit for Figure 1: G Alfthan et al, Plasma homocysteine and cardiovascular disease mortality. Lancet 1997
- Even slightly elevated Hcy level promotes tiny clots that initiate arterial damage and large clots that precipitate heart attacks and strokes. Gibson et al, 1964; Morin et al, 1991; Editorial, 1981
- Baboons infused with Hcy for 5-30 days developed arterial damage proportion to Hcy concentration. There was no correlation to cholesterol. Harker et al, 1974.
- Men with high Hcy levels had > 3 times more heart attacks than those with low Hcy levels
- Men with 3 clogged coronary arteries had higher Hcy than men with 1. Malinow MR. 1989; Stampfer et al, 1992
- Anti-atherogenic, anti-clotting benefit of fish oil may result from lowering Hcy. Olszewski AJ et al, 1993;
- Cholesterol is oxidized by Hcy to generate oxysterols (oxygenated derivatives of cholesterol) and arterial damage is proportional to the concentration of oxysterols (LDL cholesterol is only an accomplice to arterial damage through oxidative modification). Hcy also generates oxysterols in food exposed to high heat and oxygen. E.g powdered egg yolk or milk, gelatin (Hcy is the only dietary substance to do so, other than damaged fatty acids,such as trans fatty acids). Processed foods may increase oxysterol presence 1000 times higher than normal.
- Long-term vitamin B6 supplementation protects against heart problems. in 1950, Dr. Moses M. Suzman (neurologist/internist) in S. Africa started his patients on a daily dose of 100 mg B6, before they were diagnosed with heart problems. Over the course of 44 years, Dr. Suzman could not point to a single one of his tens of thousands of patients who had had a coronary spasm, cardiac arrest or stroke, and “far fewer cardiac problems than would have been expected.”In 1969, he changed the daily dose to 200 mg B6 (half as a complex) and 600 IU vitamin E. In 1972, he also added vitamin C, selenium, magnesium and other nutrients;
- Common in pregnancy
- Many peoples’ livers can not convert B6 supplement pyridoxine hydrochloride to pyridoxal phosphate (PLP) – the active form of B6 in the body.
- Consequence of methionine-rich high-animal protein diets and high dietary sugar;
- Vitamin B6 seems to protect against oxysterols – B6 has been found by several researchers to act as an antioxidant in high enough concentration, protecting against oxysterols
Overly acid-forming diet / dehydration causes inflammation
When blood is less alkaline than is ideal, it can act as an irritant to the body’s tissues. An overly-acid diet is generally the result of:
- Dehydration;
- Eating more meat and dairy, less fruit and vegetables;
- Eating more cooked food than raw;
References
Blakytny R, Harding JJ, (1992) Glycation (non-enzymic glycosylation) inactivates glutathione reductase. Biochem J.
Bucal R et al. (1993) Lipid advanced glycosylation: pathway for lipid oxidation in vivo.
Bunn and Higgins, (1991)
Ceriello P et al, (April 1996 )High Glucose Induces Antioxidant Enzymes in Human Endothelial Cells in Culture, Diabetes Vol 45.
Editorial (1981): Is vitamin B6 an antithrombic agent? Lancet
Gibson JR et al, (1964) Pathological Findings in homocystinuria, J Clin Pathol ;
Harker LA et al, (1974) Homocysteinemia: Vascular injury and arterial thrombosis, New Eng Journ Med.
Malinow MR. (1989)Risk fro arterial occlusive disease. In hyperhomocysteinemia an innocent bystander? Can. J Cardiol;
Morin RJ et al, (1991) The Role of cholesterol oxidation products in the pathogenesis of atherosclerosis, Ann Clin & Lab Sci.;
McCully. K.S. (1983), “Homocysteine theory: Development and current status. Atherosclerosis Reviews 11: 157-246.
Kumar et al, (2017)The Metabolism and significance of homocysteine in nutrition and health, Nutr Metab (Lond) PubMed
Olszewski AJ et al, (1993) Fish oil decreases serum homocysteine in hyperlipemic men, Coronary Artery Dis;
Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. (2004a) Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 109:71-77.
Rabelo et al, (2022) Homocysteine is associated with higher risks of ischemic stroke: A systematic review and meta-analysis, PLos One – PubMed
Stampfer M et al, (1992) A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA.
Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. (2002) Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 39:935-942. PubMed
Ueland, P.M. & H. Refsum (1989). “Plasma homocysteine, a risk factor for vascular disease: Plasma levels in health, disease and drug therapy,”Jour. Lab. Clin. Med. 114: 473-501.;
Universityof California Study, published Feb. 1, 2003.
Yaki, K. & S. Komura (1986), “Inhibitory effect of female hormones on lipid peroxidation,”Biochem. Int. 13: 1051-1055.
Yiamouiannis, J.(1986), Fluoride: The Aging Factor, Delaware, Ohio: Health Action Press;
Yu E, Rimm E, Qi L, Rexrode K, Albert CM, Sun Q, Willett WC, Hu FB, Manson JE. (2016) Diet, Lifestyle, Biomarkers, Genetic Factors, and Risk of Cardiovascular Disease in the Nurses’ Health Studies. Am J Public Health. 2016 Sep;106(9):1616-23. doi: 10.2105/AJPH.2016.303316. Epub 2016 Jul 26. PMID: 27459449; PMC4981798.