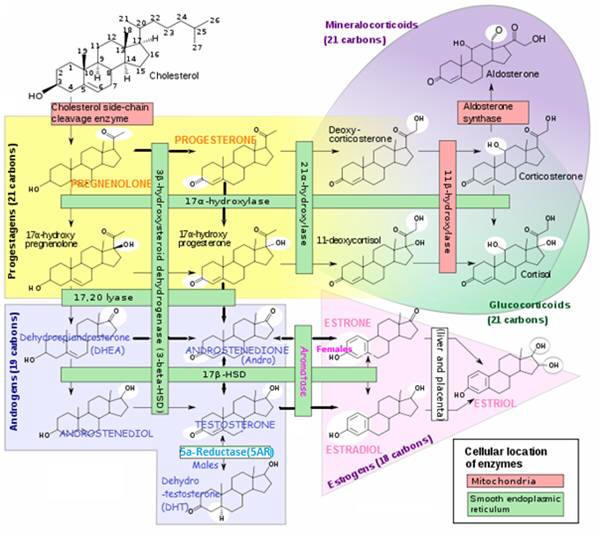
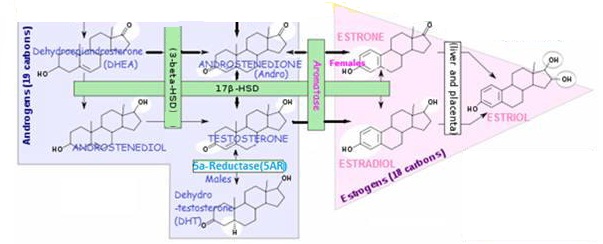
Estrogens - Predominately female sex hormones


Renowned as the feminine hormones
Estrogens are a subclass of the sex steroid hormones. Also includes androgens and progestagens
- Estrogens are responsible for breasts, hips, menstrual periods, soft skin, and a higher-pitched voice
- Estrogens also regulate the development, growth, pubertal maturation, and reproductive processes in both females and males.
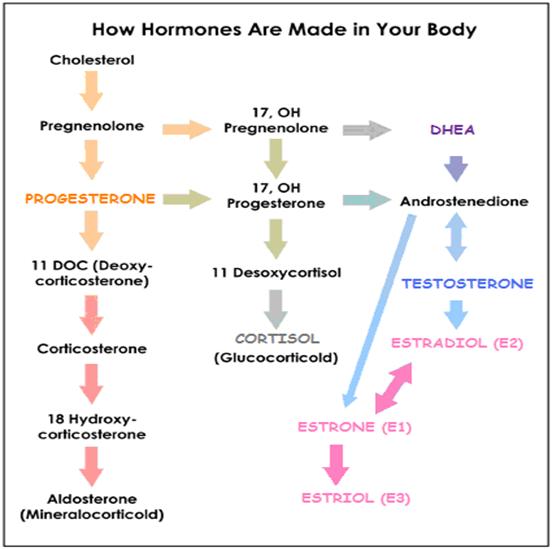
The 3 most significant estrogens are produced from androgens through the action of enzymes:
ESTRADIOL (E2, 17β-ESTRADIOL)
- The predominant form in NON-pregnant females from menarche to menopause
- The most powerful and most carcinogenic.
ESTRONE (E1)
- Produced and more predominant during menopause
- 70% less biologically active than ESTRADIOL
- ESTRONE (E1) is a significant estrogenic hormone contributor in both reproductive (0.5-1 nM) and postmenopausal (150-200 pM) women and in Men.
ESTRIOL (E3)
- Comprises nearly 80% of the total free estrogen in the female body
- Primary estrogen of pregnancy; the weakest of the three; Produced during PREGNANCY in much greater quantities than ESTRONE and ESTRADIOL- 10-100 nM in pregnant women and <7 nM non-pregnant women. Decreased ESTRIOL(E3) levels in pregnancy have been associated with complications of eclampsia and the incidence of Down’s syndrome in offspring.
Changing levels of these estrogens could be instrumental in tissue development, function, and some lifestage-specific diseases in women
Fluctuate with life stage. Suggesting specific roles for them in biological and disease processes. Fluctuations of ovarian hormones in perimenopause consist of intermittent high and low ESTRADIOL (E2 levels, followed eventually by chronically lower levels, and the predominance of ESTRONE (E1). These changes can be correlated with behavioral and other disturbances and pubertal and menstrual cycle-based fluctuations.
Produced by aromatase enzymes in non-reproductive tissues (E.g. brain, fat cells). With possible effects on non-reproductive functions.
- E.g. ESTRIOL (E3) has protective effects against the development of arthritis in certain experimental models – as demonstrated for ESTRADIOL (E2)
- These estrogens could make a difference. In the brain, bone, cardiovascular system and many other tissues during different life stages.

Health effects of estrogens
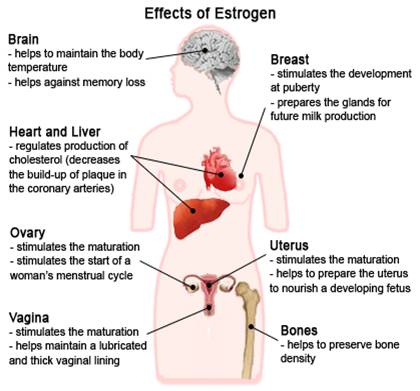
Estrogen (mainly ESTRADIOL) has numerous effects in the body | |
|---|---|
Sex Differentiation | ▪ Promotes formation of female secondary sex characteristics: growth of a girl’s sex organs, breasts and pubic hair. |
| ▪ Effeminization when estrogen to TESTOSTERONE ratio increases. E.g. Breast growth, higher voice, loss of body hair, decrease in muscle mass, increase in fatty tissue, prostate enlargement, change in sex drive; | |
Protein Synthesis | Gorski, J., A. B. Deangelo, and A. Barnea. “Estrogen action: the role of specific RNA and protein synthesis.” The sex steroids: molecular mechanisms. New York: Meredith (1971): 181-95. |
Reproductive Functions | ▪ Decreases libido (sex drive); ▪ Helps regulate menstrual cycle; ▪ Stimulates endometrial growth; ▪ increases uterine growth; ▪ ESTRIOL produced by placenta is involved with maintaining pregnancy and initiating labor. ▪ Breast-feeding stimulates estrogen production – which stimulates production of the PROLACTIN hormone to increase milk production. |
| ▪ ESTRADIOL modulates sex drive, erectile function and spermotogenesis in men – for libido, estrogen is to men what TESTOSTERONE is to women. Estrogen receptors and aromatase (enzyme that converts TESTOSTERONE to estrogen) are abundant in brain, penis, and testes, organs important for sexual function. In the brain, ESTRADIOL synthesis is increased in areas related to sexual arousal. PubMed | |
Reproductive organ cancers | ▪ Promotes hormone-sensitive reproductive organ cancers – treatment of endometrial and breast cancer involves suppression of estrogen production; ESTRADIOL promotes an oncogene, Bcl-2 (expressed at high levels, oncogenes help turn a normal cell into a tumor cell). ▪ ESTRADIOL increases expression of genes that are responsible for directing production of both androgen and estrogen hormone receptors. |
Regulates Fluid balance | ▪ Regulates salt / water retention; ▪ Increases growth hormone; ▪ Increases CORTISOL, SHBG (Sex-Hormone Binding Globulin) -binds ESTRADIOL (and TESTOSTERONE) in blood and thus inhibits their bioavailability, since only “free”hormones are active) |
Structural | ▪ Accelerates height growth; ▪ Reduces muscle mass; ▪ Maintains blood vessels /skin; |
Shortened height | ▪ Can cause early epiphseal closure near the end of puberty – thus limiting height |
Protects against heart disease / Blood Clotting | ▪ Increases HDL & triglycerides / Decrease LDL & fat deposition in coronary arteries;▪ Blood Coagulation – Increase circulating level of factors 2, 7, 9, 10, antithrombin III, plasminogen / Increase platelet adhesiveness |
GI Tract | ▪ Reduces bowel motility; ▪ Increases cholesterol in bile; |
Lungs | ▪ Promotes lung function by supporting avioli |
Preserves bone density | ▪ Helps decrease bone loss slightly – by slowing bone resorption (osteoclast activity); antagonizes the effects of PTH, minimizing the loss of calcium from bones, |
Decreases fat burning | ▪ Increases subcutaneous fat deposits/decreases fat-burning by stimulating SNS. As an Alpha 2 adrenergic agonist, estrogen increases / activates Alpha2 adrenoceptors (A2As), which inhibit fat burning (A2As react to neurotransmitters EPINEPHRINE, NOREPINEPHRINE, and isoprenaline, for a sympathetic effect; NOREPINEPHRINE is one of the body’s primary fat burning agents), but when estrogen binds to the A2A (taking NOREPINEPHRINE’s “seat”), it creates a negative feedback loop inhibiting the release of fat-burning NOREPINEPHRINE. |
Melanin | ▪ Increases pheomelanin, reduces eumelanin |
Anti-aging for Skin | ▪ Prevents decrease in skin collagen in post-menopausal women. Topical and systemic estrogen therapy with ESTRIOL increases skin collagen content / skin thickness / skin moisture (increases acid mucopolysaccharides and hyaluronic acid, and possibly maintains stratum corneum barrier function); Possible anti-wrinkle effect on elastic fibers; See: ESTRIOL-The WEAK, Protective Estrogen |

ESTRIOL, ESTRONE, ESTRADIOL comparative strengths
The physiological effects of estrogens are mediated by the intracellular estrogen receptors (ERs) . In response to , xenoestrogens and signals emanating from growth factor signalling pathways, they regulate:
- Genomic pathway initiation of gene expression (transcription) of target genes (for protein synthesis)
- Non-genomic pathway functions
Specific effects of estrogens depend on:
- ER subtype in tissue ( ER-α and ER-β). E.g. ER-α predominates inthe uterus and mammary gland, whereas ER-βhas significant roles in the central nervous, cardiovascular, and immune systems; urogenital tract, bone, kidney, and lungs;
- Number of receptors – varies by location and whether pre- or post-menopause
ESTRONE (E1) and ESTRIOL (E3) are:
- STRONG estrogens in the NON-genomic signaling pathways and functional pathways of pituitary. Watson CS, et al, 2008
- WEAK estrogens in the GENOMIC pathway (affecting nuclear transcription activity)
ESTRADIOL(E2) is:
- STRONG in the GENOMIC and NON-genomic pathways – although ESTRADIOL (E2) (in the high pM to nM range) is the physiological estrogen most often associated with GENOMIC pathways in female reproduction and the development of cancers in reproductive tissues.
- Stronger than ESTRONE (E1) and ESTRIOL(E3) in cell proliferation – E2 effects were evident at fM (Femtomolar – 1.0 fM is 1 million times smaller than a nM ) levels, whereas E1 and E3 were effective at 1 pM and 0.1 nM and above, respectively. Watson CS, 2008

Estrogen production
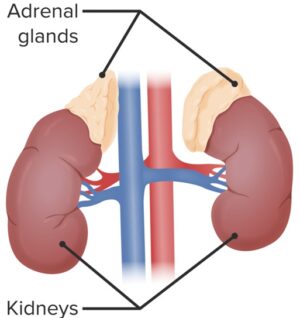
The female ovaries are not the only source of estrogen – which is also produced by the metabolic activity of certain enzymes in adrenal glands, fat, skin, and many other peripheral sites :
Estrogen production in reproductive-age women
Where is estrogen coming from in premenopausal women?
- The ovaries (follicle granulosa cells) before ovulation (follicular phase) are the main source of estrogen (predominantly ESTRADIOL, which functions as a circulating hormone
- A small amount of estrogen is produced in peripheral tissue (E.g. skin, fat, muscle) by the conversion of circulating androgens (via aromatase enzyme)
- The placenta produces ESTRIOL during pregnancy
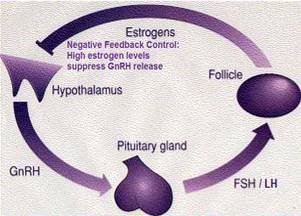
Feedback mechanism of estrogen production during reproductive cycle.
ESTRADIOL production is under the cyclical endocrine control of two PITUITARY gonadotrophins, LH and FSH, which are released by hypothalamic GnRH (which is subject to negative estrogen feed-back from the ovaries)
By negative-feedback – high-level estrogen causes a decrease in FSH / LH production by inhibiting hypothalamic GnRH (Gonadotropin Releasing Hormone) production.
FSH / LH ▲→ Estrogen ▲
Estrogen ▲▲▲ → GnRH ▼ → FSH / LH ▼→ Estrogen ▼
(Rising levels of estrogen in the blood begins follicle/egg development).
Luteinizing hormone (LH) – binds with LH receptors to stimulate follicle thecal cells to produce mainly ANDROSTENEDIONE, which is supplied to the neighboring follicle granulosa cells as a substrate with which to make ESTRADIOL;
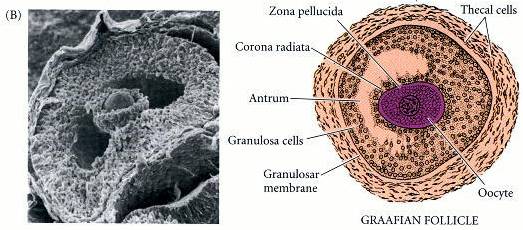
Follicle-stimulating hormone (FSH) –
- FSH stimulates granulosa cells of ovarian follicle (a collection of fluid around eggs) – (1) to stimulate growth of the immature follicle and (2) to stimulate follicle secretion of estrogens – mainly ESTRADIOL;
- Follicle granulosa cells have FSH -sensitive; aromatase activity and proliferate in response to estrogens:
- Inhibited by androgens;
- FSH regulates transcription of the aromatase gene (CYP19A1) in ovarian granulosa cells.
- Substrate ANDROSTENEDIONE is supplied by ovarian theca cells and is converted to ESTRONE by aromatase – followed by conversion of ESTRONE to ESTRADIOL ▲ by 17β-HSD enzyme.
- Estrogen stimulates the uterine endometrium to proliferate and to synthesize PROGESTERONE receptors (PR’s) ▲ in the cytosol;
- After ovulation (2nd half of menstrual cycle, called the luteal phase), estrogen ▼ production / secretion from the ovarian granulosa cells decreases;
The rise in ESTRADIOL causes expression of LH receptors ▲ on maturing follicles, which also indirectly increases ESTRADIOL ▲ production. By stimulating theca cells to provide more substrate ANDROSTENEDIONE. ESTRADIOL continues its positive feedback loop supplying LH receptors for LH activation to provide more ANDROSTENEDIONE substrate for additional estrogen production, until high estrogen levels inhibit hypothalamic GnRH, causing a decrease in fsh production and a subsequent decrease in estrogen production;
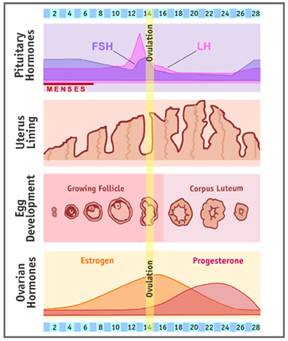
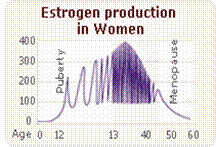
Normal female estrogen production fluctuates during reproductive years
- Normal, average estrogen levels (measured in pg/ml) vary for a woman at different times throughout her reproductive years.
Estrogen Production Site Chart | ||||
Who ? | Estrogen Names | Production Sites (by aromatase enzyme) | Aromatase produced in response to: | Comments |
| Reproductive Age Women | ESTRADIOL (E2) (17-beta ESTRADIOL)(circulating hormone) | Ovaries (granulosa cells) | FSH | ▪ ESTRADIOL functions as a circulating hormone (acting on distant target tissues) – Most abundant and potent natural form of estrogen in non-pregnant females during reproductive years. ANDROSTENEDIONE from ovarian theca cells or adrenal cortex is principal substrate for production of ESTRADIOL in ovarian granulosa cells. |
| Men and Women | ESTRONE (E1) ESTRADIOL (E2)(Localized metabolism) | Adipose tissue(mesenchymal cells) Bone (chondrocytes, osteoblasts) Skin Fibroblasts | Glucocorticoids; Class 1 Cytokines | ▪ ESTRONE (E1) is less abundant than E2 and E3 – but the only one of the 3 present in any quantity in and post-menopausal women; ▪ Adipose and Skin cell aromatization is the major source for circulating ESTRADIOL in Men and post-menopausal women -adrenal ANDROSTENEDIONE (a derivative of PROGESTERONE) is converted by aromatase to ESTRONE (E1), which is converted to ESTRADIOL by 17βhydroxysteroid dehydrogenase (17β-HSD) activity. ▪ ESTRONE is a reservoir for ESTRADIOL – ESTRONE is converted to a long-lived storage derivative, called ESTRONE sulfate, which can be converted as needed to the more active ESTRADIOL. ESTRONE is weaker than ESTRADIOL ▪ Adipose tissue is the greatest peripheral source of aromatase in both males and females contributing to ESTRADIOL production ▪ ESTRONE acts locally at production sites – Circulating estrogens are escapees from local metabolism from such as adipose tissue, brain and bone; Thus low circulating estrogen levels in postmenopausal women and men have little bearing on concentrations in estrogen-producing tissues. E.g. the estrogen responsible for a breast tumor (has up to 10x circulation levels), brain (cognitive) functions, or bone mineralization is that produced locally at those sites. |
| Brain; Vascular endothelium; Aortic smooth muscle | ||||
| ESTRADIOL (E2)(Circulating hormone) | Adrenal Cortex | ▪ Secretes small amounts of ESTRADIOL; ▪ Adrenal cortex also supplies ANDROSTENEDIONE for conversion to ESTRONE / ESTRADIOL in extragonadal sites. | ||
| Men | ESTRONE (E1) (Circulating hormone) | Testes (Leydig cells, Sertoli cells) | FSH | ▪ Responsible for at most 15-20% of total circulating ESTRADIOL; ▪ Lehdig cells convert TESTOSTERONE (produced by testes’ Sertoli cells) → ESTRADIOL to direct spermagenesis http://erc.endocrinology-journals.org/cgi/reprint/6/2/131.pdf |
| Pregnancy | ESTRIOL(E3) | Placenta | Retinoids | ▪ Only produced in significant amounts by the placenta during pregnancy |
Estrogen production in men and post-menopausal women
(1) For men and postmenopausal women, the major sources of estrogen are via aromatization at peripheral sites other than the gonads (E.g. skin, fat, muscle) from circulating androgens.
Postmenopause, a woman’s ovaries cease production of estrogens as a result of complete loss of primordial follicles, however, estrogens continue to be synthesized, at ~ 40-60% of pre-menopausal levels at these sources.
Circulating Androgens → Estrogens
Unlike the ovaries and testes which can synthesize their own androgen precursors, these sites are totally dependent on circulating androgens for conversion to estrogens. Such androgens are produced by the adrenal cortex, ovarian stroma (especially in post-menopausal women with endometrial cancer, possibly mediated by LH and INSULIN), and testis Leydig cells;
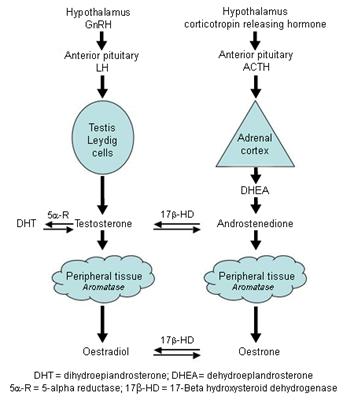
Estrogen produced at peripheral sites is probably only biologically active in local tissue. This is where the aromatase enzyme is produced to convert circulating androgens (ANDROSTENEDIONE in women) to estrogen in tissues containing aromatase, which include:
- Adipose tissue (mesenchymal cells). This is a major source of estrogen in Men (also the brain) and the major source in post-menopausal women, thus the amount of body fat significantly determines the amount of estrogen produced and stored; the breasts contain significant amounts of adipose tissue;
Tests show that a fat lady after menopause makes more estrogen than a skinny lady before menopause
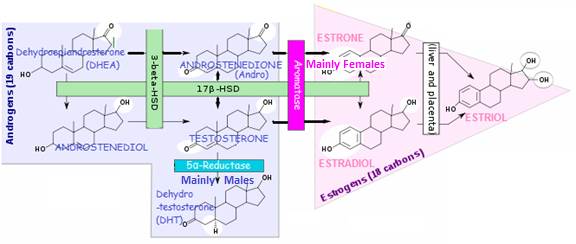
Androgens → Estrogens
- Skin. The majority of peripheral estrogen production is in the skin. Hall G and Phillips TJ, 2005
- Osteoblasts and osteoclasts in bone
- Vascular endothelial and aortic smooth muscle cells
- Brain. Including medial preoptic/anterior hypothalamus, the medial basal hypothalamus and the amygdale
- Fetal liver
(2) Small amounts are produced in the male testes
- Stimulated by LH, Lehdig cells produce androgens (TESTOSTERONE, ANDROSTENEDIONE, DHEA as substrates for FSH-stimulated production and secretion of ESTRADIOL and ESTRONE (via aromatase enzyme) by the Sertoli cells (but less ESTRADIOL is produced than by a woman’s ovaries). Only ~20% of the substrate TESTOSTERONE is secreted by the testes’ Lehdig cells.
- FSH also increases the Leydig cells response to LH by increasing the number of LH receptors expressed on Leydig cells.
Hemsell DL et al, 1975; Longcope Christopher et al, 1972
(3) To a lesser extent, estrogen can be derived from its storage form ESTRONE sulfate (E1S)
Tissues can draw on E1S as needed.

Estrogen metabolism
Enzymes are key in estrogen production
Aromatase Enzyme Converts Androgens to Estrogens
ANDROSTENEDIONE → ESTRONE (in women)
TESTOSTERONE → ESTRADIOL
Aromatase can be found in several tissues:
- Tissues having beneficial estrogen presence. Includes gonads, brain, adipose tissue (E.g. found in the breast), placenta, blood vessels, skin, bone, and endometrium.
- Health conditions where inappropriately high levels of aromatase are present in tissues. Endometriosis, uterine fibroids, breast cancer (enhanced aromatase activity found in breast fat)and endometrial cancer.
To see what substances increase or decrease aromatase production / activity:
What affects aromatase production/activity
Enzyme 17β-HSD converts ESTRONE (E1) → ESTRADIOL (E2)
- E2 synthesis occurs principally through oxidation of ESTRONE (E1). A reversible reaction;
- ESTRADIOL (E2) /ESTRONE (E1) ratio. Before menopause is > 1; After menopause E1 is the predominant estrogen;
- Tissue enzymes (E.g sulfatases and 17B-HSD) originating in bowel bacterial flora and the intestinal mucosa, activate estrogens locally to ESTRADIOL (E2) and ESTRONE (E1).
Stanczyk F. 2001.
Both ESTRADIOL (E2) and ESTRONE (E1) can be metabolized to the biologically weaker ESTRIOL (E3)
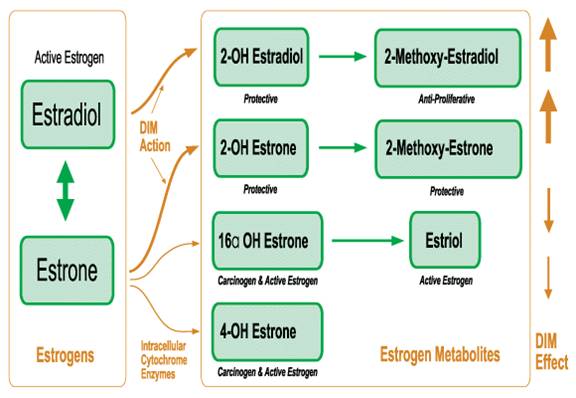
DIM Effect on Estrogen Metabolites
Estrogens are also metabolized to a number of other estrogen metabolites – some GOOD / some BAD:
- WITH estrogen-lowering dietary components (E.g. antagonists such as DIM ( a stable indole found in cruciferous vegetables), quercetin, phytoestrogens), efficient aerobic estrogen metabolism results in predominantly “GOOD” estrogen metabolites. E.g. 2-hydroxy and 2-methoxyestrogens function as antioxidants, and have the power to eliminate damaged or cancerous cells throughout the body.
- WITHOUT estrogen-lowering dietary components, INefficient anaerobic estrogen metabolism results in predominantly “BAD” estrogen metabolites. E.g. 16-hydroxy ESTRONE and 4-hydroxy ESTRONE, which function as damaging oxidants that can ultimately promote cancer.

Estrogen concentration / activity in tissues
Many factors determine estrogen concentration / activity in a particular tissue
Estrogen’s affinity (binding strength) for components of that tissue, relative to its affinity for the blood
Potency of different estrogenic compounds
Comparative strengths of ESTRADIOL / ESTRONE / ESTRIOL
Activation / inactivation of estrogen receptors.
Estrogens (like all steroid hormones) readily diffuse across cell membranes, where they bind with estrogens receptors. This leads to the production of specific proteins that express the effect of estrogen upon the target cell.
- Inactivated by PROGESTERONE
- Activated by many physical and chemical conditions
Xenoestrogens -“Gender benders”
Plasma, total, or free estrogen concentrations.
Circulating levels of estrogen
External estrogen dose
Tissue activity of enzymes which convert androgens to estrogens
Aromatase is the main conversion enzyme and can be found in all parts of the body:
- Fat and skin. Major sources of estrogen, especially in older people, since aromatase activity increases with aging.
- Also, fibroblasts, smooth muscle cells, breast and endometrium (uterine lining), pancreas, liver, brain, bone, more.
Aromatase activity (and therefore estrogen production) is affected by many factors:
- Aromatase activity is increased ▲ by aging, and under the influence of PROLACTIN, CORTISOL, prostaglandin, and the pituitary hormones FOLLICLE STIMULATING HORMONE and GROWTH HORMONE.
- Aromatase activity is inhibited ▼ by: PROGESTERONE , thyroid hormones, aspirin, and high altitude.
- Aromatase activity is involved in breast cancer, endometriosis, uterine cancer, lupus, gynecomastia. Also, many other diseases
- Aromatase in mammary tissue appears to increase estrogen receptors and cause breast neoplasia. Independently of ovarian estrogen
Metabolic clearance of estrogen
A healthy liver combines estrogen with glucuronic acid (“sugar acid”) to make it water-soluble for elimination. In the 1940’s, the Biskinds demonstrated some causes of liver impairment that decrease its ability to excrete estrogen, including:
- A dietary protein deficiency
- Hypothyroidism – Prevents the liver from attaching glucuronic acid to estrogen, and so increases the body’s retention of estrogen, which in turn impairs the thyroid gland’s ability to secrete thyroid hormone. Hypothyroidism often results from nutritional protein or iodine deficiency.
Activity of beta-glucuronidase, sulfatase and other enzymes determine whether estrogen is in inactive water-soluble or active oil-soluble form.
Water-soluble estrogen must be made oil-soluble to be able to enter cells and exert its effects through their lipid membranes.
Sulfatase
- Sulfatase. Attaches sulfuric acid, and also several other enzymes modify the activity /solubility of estrogens.
- Sulfatase activity is inhibited ▼ by: antiestrogenic hormones. (E.g. PROGESTERONE)
- Sulfatase also releases estrogen in tissues
Beta-glucuronidase (found in inflamed tissue). Normally, the healthy liver combines estrogen with glucuronic acid to make it water-soluble for elimination. However, if it passes through inflamed tissue, the large amounts of beta-glucuronidase there remove glucuronic acid, converting inactive estrogen-glucuronides into active estrogen, which accumulates in tissue
- Beta-glucuronidase activity is increased ▲ by: Inflammation.
- Beta-glucuronidase activity is inhibited ▼ by PROGESTERONE (in some tissues), and maybe more so with aging
Cristofalo VJ & Kabakjian J , 1975
- Breast cancer treatment uses glucaric acid – which inhibits beta-glucuronidase; also, glucuronic acid tends to inhibit the intracellular release of estrogen by beta-glucuronidase.
- Glucuronidase – converts water-soluble estrogen glucuronides into oil-soluble forms by attaching glucuronic acid.
PROGESTERONE prevents tissue from concentrating estrogen
- PROGESTERONE antagonizes the effect of estrogen. By reducing estrogen receptor levels. PROGESTERONE also acts indirectly, by its antagonizing action against PROLACTIN, it prevents PROLACTIN from stimulating the formation of estrogen receptors
- Tissue / plasma ESTRADIOL (E2) ratio varies with PROGESTERONE presence – ranging from 20.36 in early follicular phase to 1.45 in mid-luteal phase when PROGESTERONE level is high.
- During pregnancy, tissue estrogen decreases as blood PROGESTERONE increases.
- PROGESTERONE production sharply decreases in aging allowing tissues to concentrate estrogen even with low serum estrogen – similar to follicular phase of menstrual cycle.
- In postmenopausal women, the tissue E2 concentration is not significantly lower than in menstruating women in follicular phase. Akerlund, et al., 1981
- An excessive estrogen presence is causing an hormonal imbalance (referred to as Estrogen Dominance), especially with PROGESTERONE (in women) and TESTOSTERONE {in men) One must consider not only the absolute level of a hormone but also its relative ratios with other hormones, including ESTRADIOL, PROGESTERONE, TESTOSTERONE, CORTISOL and thyroid hormones. A “sea” of estrogen “look-alikes” have infiltrated our lives today, having an estrogenic effect that is manifesting as a wide range of health problems by causing hormonal imbalance, especially with PROGESTERONE. E.g. infertility / miscarriage, menopausal symptoms, PMS, fibrocystic breasts, ovarian cysts, hirsutism, uterine fibroids, reproductive organ cancers in men and women, enlarged prostate, osteoporosis, depression/anxiety, water retention, inflammation, and much more . Healthy women without breast cancer have saliva-tested PROGESTERONE levels routinely 200-300 times greater than saliva ESTRADIOL level. Compare this to women with breast cancer, who have a saliva PROGESTERONE / ESTRADIOL ratio considerably less than 200 to 1. When you consider the many functions of PROGESTERONE, which are being suppressed by excess estrogen, you can understand why just about every aspect of health is affected. A so-called estrogen dominance problem is addressed by first reducing estrogen exposure, attending to other important factors involved with hormone imbalance* and then possibly, by supplementing PROGESTERONE .
Xenoestrogens -“Gender benders”
PROGESTERONE – “Precursor to Androgens, Estrogens and Corticoids”

Circulating estrogen levels
In men and postmenopausal women, extragonadal sites are the major source for the LOW circulating ESTRADIOL, which is NOT a good indicator of tissue levels and is NOT the body’s main driver of estrogenic activity. In fact, estrogen circulation levels actually reflect estrogen which has escaped from metabolism at extragonadal sites of production. For example, the estrogen responsible for breast cancer development, maintenance of bone mineralization and cognitive function is not circulating estrogen, but rather that which is produced locally at these specific sites within the breast, bone and brain, respectively.
Circulating, blood ESTRADIOL levels are actually higher in men than postmenopausal women –
- In males, concentration is ~2-3 ng/dl compared to postmenopausal women at ~1.5ng/dl. –ESTRADIOL production rate in blood is also significantly higher (25-40 μg/24 h) in Men. Male levels of estrogen tend to increase with age (at the expense of TESTOSTERONE) due to increased aromatase activity
- Premenopausal womenhave an ESTRADIOL concentration of ~5.5.ng/dl in days 2-4 of menstrual cycle.
Circulating levels of pituitary and steroid hormones in postmenopausal women compared with levels in postmenopausal women studied during first week of the menstrual cycle(Days 2-4)
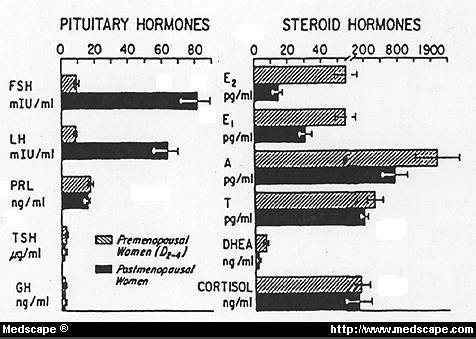
(To convert: pg/ml->Ng/dl, divide by 10)

Estrogen transport and availability
Estrogen transport
ESTRADIOL(E2) is transported in serum, reversibly bound to sex hormone-binding globulin (SHBG) and with less affinity, to albumin
Estrogen bioavailabilty
The bioavailability of estrogens is modulated by their binding to the sex hormone-binding carrier SHBG
- 40% of ESTRADIOL(E2) is bound with high affinity to SHBG
- 59% remainder is loosely attached to albumin – both the free and the albumin-bound E2 are bioavailable to various tissues.
- ~2% of E2 circulates as a free steroid
Exogenous oral estrogens stimulate increased SHBG production(in the liver cells), whereas androgens suppress SHBG levels(making E2 more bioavailable, since there is less SHBG to bind to).
- Potency of different estrogens is reflected in their ability to bind to SHBG and to stimulate its hepatic synthesis – E.g.Unconjugated equilin (one of the estrogens isolated from horse urine E.g. in Premarin), ESTRONE (E1), and ESTRADIOL(E2) bind with high but different affinities to SHBG. In a comparative study, 0.625 mg conjugated equine estrogens (CEE) increased plasma SHBG by 100% over baseline after 3 months of treatment; 1 mg of oral E2 increased plasma SHBG by 45% and 50 mcg of transdermal E2 increased it by 12%. Nachtigall LE,et al, 2000
Estrogen transport
ESTRADIOL(E2) is transported in serum, reversibly bound to sex hormone-binding globulin (SHBG) and with less affinity, to albumin
Estrogen bioavailabilty
The bioavailability of estrogens is modulated by their binding to the sex hormone-binding carrier SHBG
- 40% of ESTRADIOL(E2) is bound with high affinity to SHBG
- 59% remainder is loosely attached to albumin – both the free and the albumin-bound E2 are bioavailable to various tissues.
- ~2% of E2 circulates as a free steroid
Exogenous oral estrogens stimulate increased SHBG production(in the liver cells), whereas androgens suppress SHBG levels(making E2 more bioavailable, since there is less SHBG to bind to).
- Potency of different estrogens is reflected in their ability to bind to SHBG and to stimulate its hepatic synthesis – E.g.Unconjugated equilin (one of the estrogens isolated from horse urine E.g. in Premarin), ESTRONE (E1), and ESTRADIOL(E2) bind with high but different affinities to SHBG. In a comparative study, 0.625 mg conjugated equine estrogens (CEE) increased plasma SHBG by 100% over baseline after 3 months of treatment; 1 mg of oral E2 increased plasma SHBG by 45% and 50 mcg of transdermal E2 increased it by 12%. Nachtigall LE,et al, 2000

Body's elimination of estrogen is mainly via the liver
75-85% of estrogen inactivation / clearance is by the healthy LIVER, the rest is cleared extrahepatically. Contrast this to the androgens, which can also be significantly inactivated in other tissues;
The major route of estrogen inactivation is via the healthy liver’s conversion of estrogen to ESTRONE. ESTRONE has only ~30% of ESTRADIOL’s activity level (17BHSD in some peripheral tissues also makes this conversion). In the liver, ESTRONE is then dealt with by one of the following 3 methods:
- Formation of ESTRIOL. The major urinary estrogen in humans, ESTRIOL formation is increased ▲ with obesity and hypothyroidism, and decreased ▼ with hyperthyroidism;
- Catechol estrogen formation (liver, nerve cells, other tissues). ESTRONE and ESTRADIOL catalyzed by 2-hydroxylase to their 2-hydroxylated metabolites and excreted after processing by COMT; catecholestrogen formation is increased in hyperthyroidism ▲ / decreased in hypothyroidism ▼ ; a specific hydroxylase (CYP1B1) can activate the potentially mutagenic/carcinogenic ESTRADIOL metabolite 4-hydroxyESTRADIOL, which gives rise to radicals that can damage DNA.
- Sulfotransferase reaction (liver, uterus, other tissues). ESTRONE is converted to biologically inactive ESTRONE sulfate, the most abundant plasma estrogen, which can be metabolized to ESTRIOLand catecholestrone, but is more often conjugated with glucuronic acid (“sugar acid”), making the estrogen water soluble for easy elimination in the urine;
The liver clears 0.70 – 0.85 liters/hr Longcope C et al, 1968
Blood flow in the liver appears important in determining the overall metabolic clearance of E2. Longcope C and Tait JF, 1971
One of the best ways to keep the liver functioning efficiently is by doing a coffee enema
Coffee Enema – “A Trash Truck”
For other liver detoxification methods:

Estrogen Receptors (ERs)
About ER Recptors
Once inside the cell,an estrogenic hormone binds to estrogen receptors (ERs) to activate them
- Binding modulates the expression of many genes. Leading to formation of messenger RNA (mRNA);
- mRNA interacts with ribosomes to produce proteins that express a specific effect of estrogen upon the target cell
Two types of estrogen receptors
- ER-α receptors. Found in the endometriumuterus, breast cancer cells, ovarian stroma cells, hypothalamus, testes, dermal papilla cells (fingerprints /nourish hair follicles and bring food and oxygen to the lower layers of epidermal cells), sebaceous glands and adrenal glands.
- ER-β receptors. Discovered 1996, present in the same areas as ER- α to a degree, but also found in more diverse parts of the body, including the skin, brain, kidney, bone heart, lungs, intestinal mucosa, prostate and endothelial cells (line the interior surface of blood vessels).
Skin / Hair follicle receptors. ER-β (and not ER- α) is the main mediator of estrogenaction in human skin and the hair follicle. ER-β is highly expressed in epidermis, dermal fibroblasts, and outer root sheath of the hair. http://www.nature.com/jidsp/journal/v8/n1/full/5640100a.html
HUMAN | ER-α | ER-β | AR | PR |
| Epidermis | ER- β | |||
| Dermal fibroblasts | ER- β | |||
| Outer root sheath of hair follicle | ER- β | |||
| Dermal papilla cells | ER-α | AR | ||
| Sebaceous glands | ER-α | ER- β | AR | |
| Eccrine sweat glands | ER- β | AR | PR | |
| Adipose Tissue(subcutaneous) | ER- β |
Binding affinity of different estrogens
- ESTRADIOL binds equally well to both ER- α and ER-β
- ESTRONE binds preferentially to ER- α
- ESTRIOL (postmenopausal estrogen) and genistein (Isoflavones) bind preferentially to ER-β
ER amounts ▼ decrease significantly after menopause
ESTRADIOL increases number of ER- α and AR receptors
- ESTRADIOL stimulates production of both ER- αreceptors and androgen receptors (Ars) – promoting a greater hormonal effect for estrogen and androgens.
Also, even a low dose of the synthetic estrogen bisphenol A (prolific in our world and bodies today – see xenoestrogens) has the same effect as natural ESTRADIOL. Richter CA et al, 2007
Stimulation is blocked by estrogen receptor antagonists – E.g. PROGESTERONE , tamoxifen,
ESTRADIOL is a relatively potent androgen receptor (AR) binder; Nakia et al, 1997; Richter CA et al, 2007
Estrogen Receptors (ERs) and Cancer
Estrogen and the ERs are implicated in breast cancer, ovarian cancer, colon cancer, prostate cancer and endometrial cancer – Advanced colon cancer is associated with a loss of ER-β, the predominant ER in colon tissue, and colon cancer is treated with ER-β-specific agonists. Harris et al, 2003
A test result of ER-positive (ER+) – means that ERs are “over-expressed” and that estrogen is causing a tumor to grow. ER+ tumors are slightly slower-growing than ER- tumors, and the cancer should respond well to hormone suppression treatments.
- 0 is no receptors found,
- 1+ is a small number,
- 2+ is a medium number, and
- 3+ is a large number of receptors.
ERs and Breast Cancer. Test results show ER+ in ~ 70% of breast cancer cases
- Estrogen binding to ER stimulates proliferation of mammary cells (increases cell division/DNA replication) leading to mutations.
- Estrogen metabolism may produce genotoxic waste.
ER- α is associated with more differentiated tumors. ER-β involvement is controversial.
Different versions of the ESR1 (Estrogen Receptor 1 protein coding gene) associated with different risks of developing breast cancer. Deroo BJ and Korach KS, 2006

References
Cristofalo VJ & Kabakjian J (1975) Mech. Ageing Dev. 4, 19-28.
Deroo BJ, Korach KS (2006). “Estrogen receptors and human disease”. J. Clin. Invest. 116(3): 561-7.
Hall G, Phillips TJ. Estrogen and skin. The effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol 2005;53:555-68.
Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC (2003). “Evaluation of an estrogen receptor-beta agonist in animal models of human disease”. Endocrinology 144 (10): 4241-9 PubMed
Hemsell DL et al, Plasma precursors of estrogen II. Correlation of the extent of conversion of plasma ANDROSTENEDIONE to ESTRONE with age. J. of Clin End. And Met, 1974;
Longcope C, Layne DS, Tait JK. Metabolic clearance rates and interconversions of estrone and 17beta-ESTRADIOL in normal males and females. J Clin Invest. 1968 Jan;47(1):93-106. PubMed
Longcope C, Tait JF J Clin Endocrinol Metab. 1971 Apr;32(4):481-90.PubMed
Longcope Christopher et al, The secretion of ESTRONE and ESTRADIOL-17βby human testis, Elsevier Science Inc., 1972
Leslie Baumann. Cosmetic Dermatology: Principles and Practice, Second EditionMcGraw Hill Professional,Apr 8, 2009
Nachtigall LE,Raju U,Banerjee S,Wan L,Levitz M.Serum ESTRADIOL-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, ESTRADIOL, and estrone levels. Menopause. 2000 Jul-Aug;7(4):243-50. PubMed
Nakla AM, Romas NA, Rosner W. ESTRADIOL Activates the Prostate Androgen Receptor and Prostate-specific Antigen Secretion through the Intermediacy of Sex Hormone-binding Globulin March 14, 1997 The Journal of Biological Chemistry,272, 6838-6841. Jbc Abstract
Richter CA, JA Taylor, RL Ruhlen, WV Welshons and FS vom Saal. 2007. ESTRADIOL and bisphenol a stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells,. Environmental Health Perspectives, in press. Link
Stanczyk F. Estrogens used for replacement therapy in postmenopausal women. Gynecol Endocrinol. 2001;15(suppl 4):17-25.
Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of ESTRADIOL compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008 Sep;22(9):3328-36. doi: 10.1096/fj.08-107672. Epub 2008 Jun 9. PubMed