I3C (=> DIM) in crucifer vegetables - Estrogen blocker with anti-cancer benefits


DIM is derived from crucifer vegetables
I3C from cruciferous vegetables is converted to DIM
The compound I3C (Indole-3-carbinol) is derived from cruciferous vegetables when plant cells are chopped or chewed. By the hydrolysis (breakdown) of glucobrassicin (an indole glucosinolate) via the myrosinase enzyme. The members of the Brassica family include broccoli, brussels sprouts, bok choy, kale, chard, mustard greens, cauliflower, cabbage, rutabaga and turnips.
In the acidity of the stomach, I3C is converted to various active reaction products, including diindolylmethane (DIM) , which increases the conversion of ESTRADIOL to a “weaker” estrogen (2OHE). DIM induces certain enzymes in the liver to block the production of the toxic estrogens and step up the production of the beneficial forms.
The various reaction products are responsible for the activity attributed to I3C, BUT NOT ALL of them have a positive effect on health problems. The alkaline environment of the intestine is less likely to form reaction products.
- DIM (dimer 3,3′-diindolylmethane) is one of I3C’s beneficial reaction products. ~ 10-20% of I3C consumed is converted to DIM.
To protect I3C levels, the best way to eat crucifers is lightly steamed or even better – pickled. Boiling brassica vegetables deactivates the myrosinase enzyme, but a lesser amount of I3C is still formed (via glucobrassin hydrolysis by bacteria in the intestines)
Helpful for those with “allergies”, asthma, chronic fatigue, or MCS.

NATURAL way to obtain quantities of I3C is by eating broccoli sprouts
The natural way to obtain I3C is to consume it in the whole foods that contain it. E.g by eating broccoli. However, broccoli sprouts are even better, since they contain hundreds of times more I3C than broccoli.
Broccoli sprouts can be found at many health food stores, certain grocery stores (E.g. Publix), also some farmer’s markets. If not available ready-grown, purchase organic broccoli seeds and sprout your own; they can be added to salads and sandwiches, but to consume in quantity, they can be lightly steamed and eaten as a vegetable – they taste similar to steamed spinach.
Using I3C as an ISOLATED supplement is NOT recommended due to its mixed study results (detailed on this page). The brassica vegetables contain many phytonutrients, which could be responsible for their generally favorable effect on health and with cancer. In particular the nutrient sulfuraphane:

DIM supplementation is a useful anti-estrogen / anti-cancer "tool"
The reaction product DIM is considered a safe and effective supplement used as an estrogen-blocker and for its anti-cancer effects. Having benefits and safety properties not attributed to an I3C supplement. Estrogen can be broken down to both “good” metabolites (E.g.2-hydroxy estrogen) and “bad” i.e. carcinogenic metabolites (E.g. 4-hydroxy estrogen and 16-hydroxy estrogen) – DIM supplementation promotes more of the “good” metabolites and less of the “bad”, which are affiliated with breast cancer and other health problems
DIM particularly seems to be a worthwhile supplement for:
- Any estrogen-sensitive (E.g. reproductive cancers, lung cancer) or HPV-related cancer (E.g. squamous cell lung cancer)
- Losing weight resulting from estrogen-related weight gain
The I3C reaction product DIM showing good results with cancer
- The original discoverer of the importance of cruciferous indoles for breast health advocates DIM over I3C – Leon Bradlow, Ph.D. now supports and advocates absorbable DIM, and not I3C, as the preferred supplement, even though he initiated and performed the majority of human and animal testing of I3C.
- DIM cannot be converted to undesirable ICZ in vivo
- DIM blocks dioxin receptor. Dioxin is a well-known carcinogen Chen I et al, 1996
- DIM is not estrogenic or a growth promoter of cancer cells. DIM exerts its control over cancer growth without inducing unwanted enzymes and their production of highly estrogenic16OHE1 from I3C
- “DIM is a more effective inducer of apoptosis than I3C” Chen DZ et al, 2001
- DIM controls growth in experimental breast cancer. Kang JS et al, 2001; Chen I et al, 1998;
NOTE that Cell Culture studies showing DIM as a contributor to breast cancer cell growth were done in an artificial estrogen–free environment never seen in vivo. In the article “DIM and I3C: The Real Facts on Safety” by Michael A. Zeligs, M.D., he writes:
” In the presence of estrogen, DIM is consistently anti-proliferative and reduces the growth signal provided by estrogen (2). This includes growth-inhibiting activity in both estrogen receptor positive and negative cancer cell types (3). The estrogen-independent activation pathway described by Riby et al (1) involves the increased phosphorylation of estrogen receptor proteins, and not the direct interaction of DIM with the estrogen receptor. This action may actually be of benefit in cancer control. Understood in context, this receptor activation pathway contributes to the control of abnormal cell growth in living animals through enhanced “programmed-cell-death” (apoptosis) and a reduced tendency for the metastatic spread of cancer (4). Unlike ICZ, LTR, CTR or ASG, DIM exerts its control over cancer cell growth without activating the dioxin receptor or inducing unwanted enzymes.Direct control over cancer cell growth by DIM has now been shown in breast, uterine, cervical, ovarian, and colon cancer cells.I3C has been noted to control cancer cell growth in these cell types as well. However, in cell culture media at 37 degrees Centigrade, most of the I3C is converted to DIM in 24 hours. Since no ICZ and the other condensation products formed in gastric acid occur in cell culture media, DIM may very well be responsible for much of the activity attributed to I3C in prior cell culture studies”. Riby JE et al, 2000; Chibo et al, 2000; Chibo et al, 2001; Platet N et al, 2000
Studies Supporting I3C / DIM Anti-Cancer Action | ||||
| Cancer Type | I3C or DIM | Study Findings | StudyType# | Study |
| Prostate | I3C | Induces apoptosis in prostate cancer cells | C | PubMed |
| Breast | DIM | Induces apoptosis in breast cancer cells | C | PubMed |
| Breast | I3C | Induces apoptosis in breast cancer cells | C | PubMed |
| Cervical | Estrogen protects cervical cancer cells from apoptosis | C | PubMed | |
| Breast | I3C* | INHIBITS cancer development | A | PubMed |
| Uterus | I3C* | INHIBITS cancer development | A | PubMed |
| Stomach | I3C* | INHIBITS cancer development | A | PubMed |
| Colon | I3C* | INHIBITS cancer development | A | |
| Colon | I3C | Induced cell-cycle arrest and apoptosis in human colon cancer cells | C | PubMed |
| Lung | I3C* | INHIBITS cancer development | A | PubMed |
| Liver | I3C* | INHIBITS cancer development | A | PubMedPubMed |
| Breast | I3C | Interferes with the breast cancer cell cycle ^; also had an indirect role in selective apoptosis-inducing ability in premalignant and malignantbreast epithelial cells | C | PubMed |
| Breast | I3C | Revealed an important connection between I3C and the mammalian gene Cdc25A, necessary for cells to divide and proliferate; When a vegetable compound containing I3C was given orally to lab mice, it dramatically reduced tumor size up to 65 % | A | Cancer Prevention Research, online pubn.Jun 29, 2010 |
# Study Type is Cultured (C), Animal(A) or Human (H)
*Administered BEFORE or AT THE SAME TIME as a carcinogen (required to cause cancer in most animal models)
^In what may be a previously unknown signaling pathway in the cell, its unusual mechanism promotes a sharp drop in production of the gene CDK6 (cyclin-dependent kinase 6) which encodes for the CDK6 enzyme, important in the cell’s growth cycle.
% Cdc25A occurs at abnormally high levels in cancers of the breast, prostate, liver, esophagus, endometrium and colon, and in non-Hodgkin lymphoma; it is also elevated in other diseases, including Alzheimer’s disease. The Ohio State researchers discovered that I3C causes the destruction of Cdc25A, resulting in a sudden halt to breast cancer cell growth.
About half of breast cancer cases have abnormally high levels of Cdc24A, which is associated with a poor prognosis.”I3C can have striking effects on cancer cells”, according to Dr. Zou, one of the study’s research team, “and a better understanding of this mechanism may lead to the use of this dietary supplement as an effective and safe strategy for treating a variety of cancers and other human diseases associated with the overexpression of Cdc25A.”
I3C / DIM are likely to benefit prostate cancer and other hormone-sensitive cancers. Which have similar contributing factors.
I3C has been used for some benign cancers and abnormal growths: Voicebox and respiratory tract tumors caused by a virus, and cervical dysplasia (abnormal growth in cervix)
Unlike Tamoxifen, I3C / DIM work independently of the hormone estrogen. I3C (or its reaction product DIM) could be used as a complimentary treatment with drugs that do interfere with estrogen.
Cancer prevention. Researchers are particularly interested in supplementation of I3C or its reaction products, such as DIM, for prevention of breast, cervical, endometrial and colectoral cancers
Studies NOT Supporting I3C / DIM Anti-Cancer Action | ||||
| Cancer Type | I3C or DIM | Study Findings | StudyType# | Study |
| Breast | I3C | Shown INeffective in controlling experimental cancer growth in rats; First study concluded that, over time, IC3increased carcinogenic estrogen metabolites (E.g. 4-OH and 16-OH), which may be counteracting those of the less estrogenic 2-OH metabolites | A | PubMed PubMed+ |
| Liver | I3C | I3C promoted or enhanced cancer development when administered chronically to rainbow trout AFTER administration of cancer promoting carcinogen (post-initiation). | A | PubMed+ PubMed + |
| Breast, liver, colon | I3C | continuous I3C diet for 25 weeks did not alter tumor incidence or multiplicity among surviving rats | A | PubMed+ PubMed+ PubMed+ |
Liver, Thyroid | I3C | Enhanced cancer development in rats | A | PubMed+ |
| Colon | Enhanced cancer development in rats; second study states I3C increased proliferation and decreased apoptosis | A | PubMed PubMed+ | |
| Uterus | I3C | study concluded that excess increased activityof carcinogenic 4-OH estrogen metabolites compared to 2-OH metabolites in the liver contributed to increased uterine uterine adenocarcinomas and/or multiplicities of uterine proliferative lesions; study showed no effect on 16-OHactivity. | A | PubMed+ |
# Study Type is Cultured (C), Animal(A) or Human (H)
+ Administered post-initiation i.e. after carcinogen (required to cause cancer in most animal models)
I3C reaction products have been shown to reduce immune system natural killer cell activity in rats. Exon JH, South EH, 2000
I3C reaction products associated with reproductive toxicity in rats –similarly to dioxin. Wilker C, Johnson L, 1996; Larsen-Su SA, Williams DE, 2001
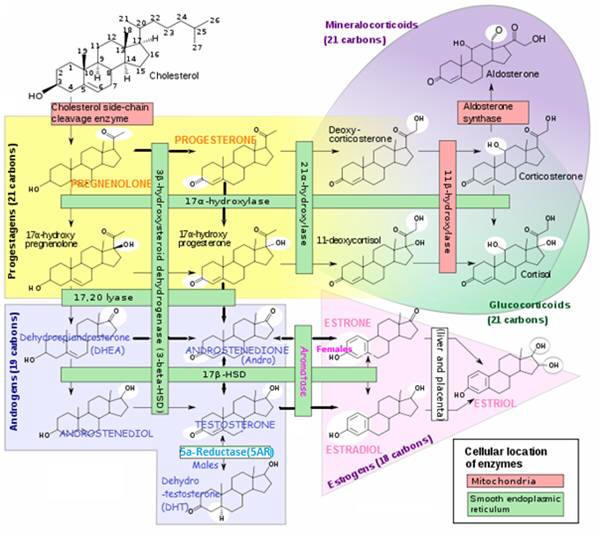
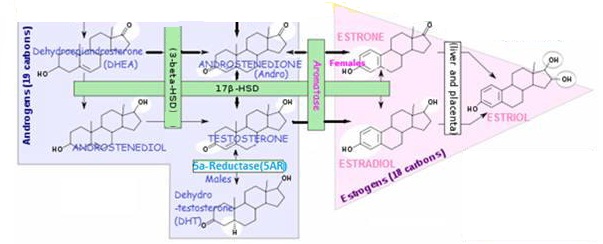
DIM is neither estrogenic or a growth promoter of cancer cells. DIM exerts its control over cancer growth by promoting production of beneficial 2OHE1 estrogen, whilst inducing less of the unwanted enzymes that produce highly estrogenic and carcinogenic16OHE1from I3C; (see below: IC3/DIM and Hormone Balancing /Alterations in Estrogen Activity and Metabolism)
Other I3C reaction products have mixed effects
- Linear Trimer (LTR). Anti-estrogenic, but activates dioxin receptor
- Cyclic Trimer (CTR). Directly activates the estrogen receptor, powerfully stimulates breast cancer cell growth, whether or not estrogen is present;
- Indole-3-acetonitrile (IAN)
- Shown protective in animal model of stomach cancer, but it has also been found to be mutagenic and to produce DNA-damaging reaction products in the presence of nitrates (common in foods). Yamashita K et al, 1988; Wakabayashi K,, et al, 1985
- IAN also found to activate inflammation-causing leukotrienes in cell membranes (via arachadonic fatty acid cascade)

The I3C reaction product ICZ (indolocarbazole) is generally undesirable.
- ICZ has both antiestrogenic and estrogenic activities. It down-regulates estrogen receptors, but is itself estrogenic, promoting enzymes which produce the carcinogenic metabolite 4-hydroxy estrogen associated with uterine tumors
- ICZ activates the dioxin receptors just like dioxin. Although dioxin has toxic properties, it is a powerful estrogen activity inhibitor, as is ICZ. ICZ is more quickly metabolized than dioxin, but has been shown to damage the thymus gland and immune system and to damage DNA. d’Argy Ret al, 1989; Park JY et al, 1996;
- Another I3C condensation product, ascorbigen (ASG) is converted to ICZ in the lower intestine. In the presence of vitamin C (naturally abundant in cruciferous vegetables), ASG is the most plentifully produced reaction product from I3C, occurring in the stomach.Some of this ASG is converted to ICZ in the lower intestine, making it available for unwanted enzyme production to promote carcinogenic 4-hydroxy estrogen. Preobrazhenskaya MN et al, 1993
- DIM cannot be converted to the undesireable ICZ in vivo
Maybe other ingredients in cruciferous vegetables are responsible for their hypothesized lower risk for some types of cancer in epidemiological studies. Brassica are relatively good sources of other phytonutrients including vitamin C, folate, selenium, carotenoids, fiber and other glucosinolates, in addition to I3C, that may be hydrolyzed to a variety of potentially protective isothiocyanates (E.g. Sulforaphane -“Eat Your Broccoli”). Verhoeven DT et al, 1996

I3C/DIM and hormone balancing /alterations in estrogen activity and metabolism
“Good” and “Bad” estrogens at work in the body
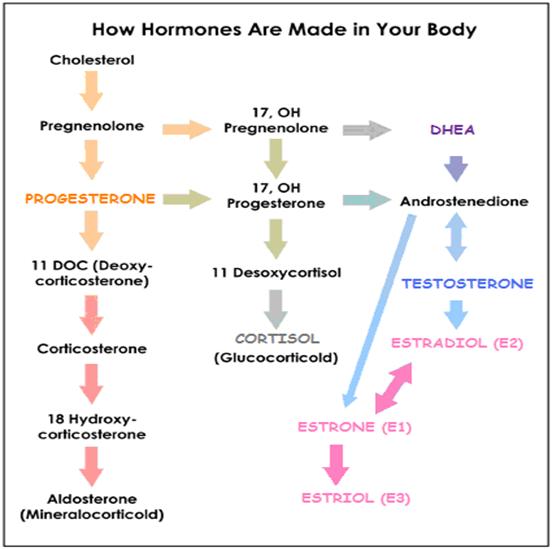
Estrogens must be removed from the body when they have finished their work. Generally, estrogens positively promote the tissue growth predominately underlying female maturation. Ideally, once their job is done, they are promptly metabolized and removed from the body. If this is not efficiently achieved, they can hang around and wreak havoc. Removal of hormones is achieved in two main ways:
- Physically removed viaurination and bowel movements
- Metabolized ( Chemically altered ) mainly by the liver to prepare them for removal – requires certain nutrients and is achieved by two main pathways:
(i) CARCINOGENIC 16 alpha-hydroxylation pathway – more active in women with estrogen-sensitive cancers; produces the carcinogenic estrogen metabolite 16alpha-hydroxyestrone (16OHE1), which is highly estrogenic and has been found to stimulate the proliferation of several estrogen-sensitive cancer cell lines. Telang NT et al, 1992; Yuan F et al, 1999;
(ii) PROTECTIVE 2 hydroxylation pathway – produces the cancer-protective estrogen metabolite 2-hydroxyestrone (2OHE1).
What factor(s) determine whether estrogen is metabolized by the carcinogenic or protective pathway?
Removal of hormones is negatively impacted by low thyroid function. Explains the link between hypothyroidism and breast or prostate cancer.
Can depend on our genetic expression, diet, lifestyle and elimination habits. E.g. a deficiency in B vitamins (B6 in particular) and/or Magnesium reduces liver’s estrogen clearance rate.
Several tactics can be used to reduce unwanted estrogen presence in the body. E.g. A coffee enema will stimulate bile production to cleanse liver. Also attend to B6 / Magnesium deficiencies
Many of estrogen’s risks can be related to a lack of its beneficial metabolites and too many of its carcinogenic metabolites
It is now known that a lower risk of future breast cancer is associated with high beneficial 2-hydroxy estrogen levels and low carcinogenic 16OHE1. Which is prone to behave like “super-estrogen” and its higher levels create a particularly carcinogenic form of estrogen dominance which can result in:
- Mutations, abnormal growth (as in cervical dysplasia). Sepkovic DW et al, 1995
- Increased risk of breast cancer. Muti P, et al., 2000
Overproduction of 16OHE1 is also seen in:
- Obesity. Hershcope RJ, Bradlow HL, 1987
- High-fat diets. Musey PI et al, 1987
- Exposure to “estrogenic” environmental chemicals (xenoestrogens). Bradlow HL et al, 1995
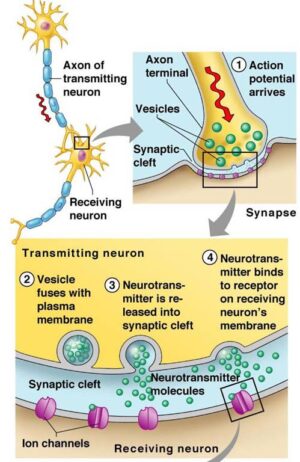
Endogenous estrogens exert their estrogenic effects by binding to estrogen receptors (ERs). Within the nucleus, the estrogen-ER complex can bind to DNA sequences(estrogen response elements/EREs) in estrogen-responsive genes (stimulated by the ligand-activated transcription factor Aryl hydrocarbon receptor/AhR) and thereby enhance transcription of these estrogen-responsive genes. ER-mediated effects that promote cellular proliferation in the breast and uterus can increase the risk of developing estrogen-sensitive cancers.
Effect of I3C/DIM on estrogen metabolism and receptor activity
- IC3 /DIM creates more “good” estrogen and less “bad” estrogen. In controlled clinical trials, oral supplementation with:
- 300-400 mg/day of I3C has consistently increased urinary 2OHE1 levels or urinary 2OHE1 : 16OHE1 ratios in women. McAlindon TE et al, 2001; Bradlow H et al, 1994; Michnovicz JJ, 1998; Michnovicz JJ et al, 1997; Wong GY et al, 1997; Reed GA et al, 2005;
- 108 mg/day of DIM increased urinary 2OHE1 levels in postmenopausal women. Meaning the body was producing them. Dalessandri KM et al, 2004
- Oral I3C supplementation can increase production of dangerous estrogen metabolites at the same time it increases the beneficial 2-hydroxy metabolites. Animal studies demonstrate unwanted enzyme-inducing effects from oral I3C supplementation. Ritter CL et al, 2001
- I3C/DIM downgrade estrogen receptor activity. In culture, I3C has been found to INHIBIT the transcription of estrogen-responsive genes stimulated by ESTRADIOL. Also, it was found that DIM binds and activates the Ah receptor (AhR) with an anti-estrogenic and anti-tumorigenic effect; it may inhibit the transcription of estrogen-responsive genes by competing for coactivators or increasing ER degradation. Chen I et al, 1998; Meng Q et al, 2000
- In contrast, a RAINBOW TROUT model found that DIM enhanced the transcription of estrogen-responsive genes. Shilling AD et al, 2001;

How to supplement DIM
As a supplement, DIM is generally considered safer and more effective than I3C
I3C is a break-down product of cruciferous vegetables. DIM is a health-beneficial reaction product of I3C formed in the stomach and intestines
Even very high doses of DIM are well-tolerated. Human studies, often at 10 times the typical supplement dose of DIM which benefits estrogen metabolism, have reported no side effects, unlike I3C, where higher doses produce side effects such as dizziness and unsteady gait. Rosen CA et al, 1998
May interfere with the breakdown of some drugs in the liver. This could lead to increased or decreased absorption of certain drugs, as well as increased side effects. Ask your health care provider about possible drug interactions with DIM if you take any prescription medication.
DIM balances hormones and aids in the breakdown of estrogen
DIM supplement needs to be formulated to be absorbable
Use of absorbable DIM has been shown effective in amounts close to that obtainable from our diet (0.3 mg/kg/day of DIM). Zeligs MA, 2002
Choose a DIM supplement where the label indicates that it has been formulated in such a way as to increase its absorption in the body. The enhanced-absorption, micro-encapsulated form patented by Bio-Response is probably the best choice, available under various labels, such as:
- Nature’s Way “DIM-plus”
Dose range of DIM (not including the absorption-aid ingredients) for hormonal balance:
- Women – 25 – 50 mg per day of DIM
- Men – 50 – 100 mg /day of DIM .
1 ½ pounds of cruciferous vegetables produces approx. 10-30 mg of DIM

References
Bradlow HL, Michnovicz JJ, Halper M, Miller DG, Wong GY, Osborne MP. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev. 1994;3(7):591-595. PubMed
Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R.Effects of pesticides on the ratio of 16/2-hydroxyestrone’a biologic marker of breast cancer risk.Environ Health Perspect. 1995; 103(SuppI7): 147-150.
Chen DZ, Qi M, Auborn KJ, Carter TH, “Indole-3-Carbinol and Diindolylmethane Induce Apoptosis of Human Cervical Cancer Cells and in Murine HPV16-Transgenic Preneoplastic Cervical Epithelium.” J Nutr. 2001 Dec;131(12):3294-3302.
Chen I, Safe S, Bjeldanes L. “Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells.” Biochem Pharmacol. 1996 Apr 26;51(8):1069-76.
Chen I, McDougal A, Wang F, Safe S”Aryl hydrocarbon receptor-mediated antiiestrogenic and antitumorigenic activity of diindolylmethane.” Carcinogenesis 1998 Sep;19(9):1631-9.Chibo Hong, Gary L. Firestone, and Leonard F. Bjeldanes, “3,3’Diindolylmethane (DIM), a dietary indole, has multiple cell suppressive effects on MCF-7, human breast cancer cells.” The American Society for Cell Biology, Fortieth Annual Meeting, December 2000, San Francisco, California.
Chibo Hong, Gary L. Firestone and Leonard F. Bjeldanes. “Induction of apoptosis in MCF-7 and MDA-MB-231 human breast cancer cells by 3,3′-diindolylmethane (DIM).” Experimental Biology 2001, March 31-April 4, 2001, Orlando, Florida.
Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161-167. PubMed
d’Argy R, Bergman J, Dencker L, “Effects of immunosuppressive chemicals on lymphoid development in foetal thymus organ cultures.” Pharmacol Toxicol. 1989 Jan;64(1):33-8.
Exon JH, South EH, “Dietary indole-3-carbinol alters immune functions in rats.”J Toxicol Environ Health A. 2000 Feb 25;59(4):271-9.
Hershcope RJ, Bradlow HL.Obesity, diet, endogenous estrogens, and the risk of hormone-sensitive cancer.Am J Clin Nutl: 1987;45(1 Supp1):283-289.
Kang JS, Kim DJ, Ahn B, Nam KT, Kim KS, Choi M, Jang DD. “Post-initiation treatment with Indole-3-carbinol did not suppress N-methyl-N-nitrosourea induced mammary carcinogenesis in rats.” Cancer Lett. 2001 Aug 28;169(2):147-54.
Larsen-Su SA, Williams DE, “Transplacental Exposure to Indole-3-carbinol Induces Sex-Specific Expression of CYP1A1 and CYP1B1 in the Liver of Fischer 344 Neonatal Rats.” Toxicol Sci. 2001 Dec;64(2):162-8.
McAlindon TE, Gulin J, Chen T, Klug T, Lahita R, Nuite M. Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity. Lupus. 2001;10(11):779-783. PubMed
Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000;130(12):2927-2931. PubMed
Michnovicz JJ. Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int J Obes Relat Metab Disord. 1998;22(3):227-229. PubMed
Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89(10):718-723. PubMed
Musey PI, Collins DC, Bradlow HL, Gould KG, Preedy JR.Effect of diet on oxidation of 17-Beta-ESTRADIOL in vivo.J Clin Endoc Metab. 1987;65:792-795.
Muti P, et al. Metabolism and risk of breast cancer:A prospective analysis of 2:16 hydroxyestrone ratio in premenopausal and postmenopausal women.Cancer Epidemiol Biomarkers Prev, 2000; in press.
Park JY, Shigenaga MK, Ames BN, “Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole [ICZ] is associated with oxidative DNA damage,” Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2322-7.
Platet N, Cunat S, Chalbos D, Rochefort H, Garcia M.”Unliganded and liganded estrogen receptors protect against cancer invasion via different mechanisms.” Mol Endocrinol. 2000 Jul;14(7):999-1009.
Preobrazhenskaya MN, Bukhman VM, Korolev AM, Efimov SA, “Ascorbigen and other indole-derived compounds from Brassica vegetables and their analogs as anticarcinogenic and immunomodulating agents.” Pharmacol Ther. 1993 Nov;60(2):301-13.
Reed GA, Peterson KS, Smith HJ, et al. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1953-1960. PubMed
Ritter CL, Prigge WF, Reichert MA, Malejka-Giganti D, “Oxidations of 17 beta-ESTRADIOL and estrone and their interconversions catalyzed by liver, mammary gland and mammary tumor after acute and chronic treatment of rats with indole-3-carbinol or beta-naphthoflavone.” Can J Physiol Pharmacol. 2001 Jun;79(6):519-32.
Rosen CA; Woodson GE; Thompson JW; Hengesteg AP; Bradlow HL, “Preliminary results of the use of indole-3-carbinol for recurrent respiratory papillomatosis,”Otolaryngol Head Neck Surg 1998 Jun;118(6):810-5.
Sepkovic DW, Bradlow HL, Ho G, et al. Estrogen metabolite ratios and risk assessment of hormone related cancers:assay validation and prediction of cervical cancer risk.Ann NY Acad Sci. 1995; 768:312-316.
Shilling AD, Carlson DB, Katchamart S, Williams DE. 3,3′-Diindolylmethane, a major condensation product of indole-3-carbinol, is a potent estrogen in the rainbow trout. Toxicol Appl Pharmacol. 2001;170(3):191-200. PubMed
Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84(8):634-638. PubMed
Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733-748. PubMed.
Wakabayashi K, Nagao M, Ochiai M, et al., “A mutagen precursor in Chinese cabbage, indole-3-acetonintrile, which becomes mutagenic on nitrite treatment.” Mutat Res 1985; 143(1-2):17-21.
Wilker C, Johnson L, Safe S, “Effects of developmental exposure to indole-3-carbinol or 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive potential of male rat offspring.” Toxicol Appl Pharmacol. 1996 Nov;141(1):68-75.
Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28-29:111-116. PubMed
Yamashita K, Wakabayashi K, Kitagawa Y, Nagao M, Sugimura T. “32P-postlabeling analysis of DNA adducts in rat stomach with 1-nitrosoindole-3-acetonitrile, a direct-acting mutagenic indole compound formed by nitrosation.” Carcinogenesis. 1988 Oct;9(10):1905-7.
Yuan F, Chen DZ, Liu K, Sepkovic DW, Bradlow HL, Auborn K. Anti-estrogenic activities of indole-3-carbinol in cervical cells: implication for prevention of cervical cancer. Anticancer Res. 1999;19(3A):1673-1680. PubMed
Zeligs MA, Sepkovic DW, Manrique CA, Macksalka M, Williams DE, Bradlow HL, “Absorption-enhanced 3,3-Diindolylmethane: human use in HPV-related, benign and pre-cancerous conditions.” American Association of Cancer Research, (42) 2002, #103483.












![<span class="ACETYLCHOLINE">Acetylcholine (Ach)</span><span class="hdg-smaller"> – Movement, memory, learning neurotransmitter</span> – [Cloned #60543]](https://healthhappening.com/wp-content/uploads/2025/12/Ach.jpg)