You on Steroids! - Control metabolism, inflammation, immunity, water-balance, sexual characteristics
- HORMONES CHART – Type, source and effect
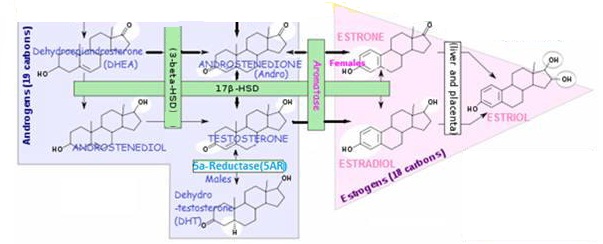
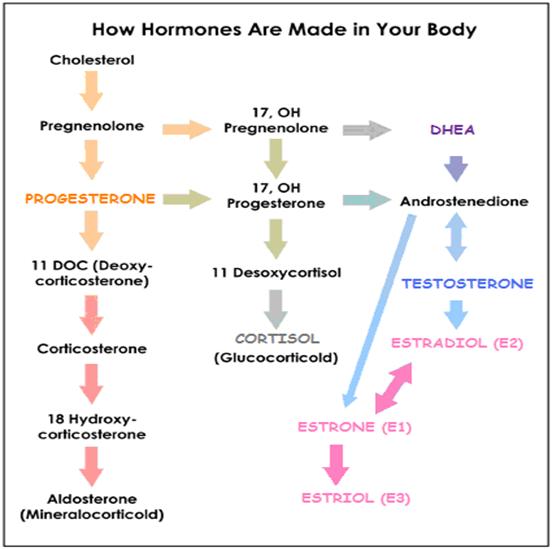
- Steroid hormone production
- Most circulating steroid hormones are bound to steroid hormone binding carrier proteins
- Steroid hormones act on target cells via receptors located in the cell interior
- Steroid hormone excretion
- Why are steroid hormone conversion enzymes important ?
Introduction to steroid hormones
Steroid hormones are a subset of the body’s hormones – Lipids derived from cholesterol. Steroid hormones include both those produced by the body and synthetically-produced in an attempt to mimic the naturally produced steroids.
What do they do?
- Steroid hormones help control metabolism, inflammation, immune functions, salt and water balance and are involved in the the development of sexual characteristics and the ability to fight illness and injury.
- Their two main functions are as signaling molecules and as components of cell membranes that alter membrane fluidity
The steroid hormones have the following structure as their nucleus:
- Small, non-water soluble molecules that must be transported in the blood bound to a protein serum globulin. Some hormones are transported by specific transport proteins, for example, sex hormone binding globulin (SHBG) binds and transports TESTOSTERONE and ESTRADIOL;
- Being fat-soluble, they can easily pass through the cell membrane. They can then combine with a protein receptor in either the cytoplasm or nucleus, depending on the hormone, to cause changes within the cell.
Steroid Class | C-?* Enzyme | Includes |
Estrogens | C18 | ESTRADIOL, ESTRONE, ESTRIOL |
Progestagens | C19 | PROGESTERONE , PREGNENOLONE |
Androgens | C21 | TESTOSTERONE, DHEA, ANDROSTENEDIONE, DHT |
Mineralocorticoids | C21 | ALDOSTERONE |
Glucocorticoids | C21 | CORTISOL |
+ Vitamin D derivatives** | D3 |
* Refers to # of carbons in molecule skeleton
** Qualify as hormones, since they are: (a) made in certain cells, (b) carried in the blood(c) affect gene transcription in target cells; technically they are sterols not steroids

Steroid hormone production
Where are steroid hormones produced?
- The steroid hormones are secreted by the ovaries (ovarian follicle /Corpus luteum), testes, prostate, and adrenal cortex – in addition, further conversions, break-downs and secretions occur inthe liver, extragonadal sites (E.g. adipose tissue, skin fibroblasts, bone, placenta , other target tissues).
How does the body make steroid hormones?
- Steroid hormones are synthesized as needed using cholesterol (mostly stored intracellularly in the tissue of origin) as the ultimate precursor, in response to appropriate signals (Luteinizing Hormone-stimulated signal transduction – the intracellular biochemical reactions that occur after stimulation of the LH membrane-bound receptor up until the initiation of cholesterol transport to the mitochondria) – the precursor is moved from storage depots to the mitochondria and smooth endoplasmic reticulum, where a series of enzymes (E.g, isomerases, dehydrogenases) rapidly convert the molecule to the appropriate steroid hormone.
- The identity of the final hormone is determined by the specific enzymes expressed in the tissue
- Limiting factor for steroid hormone secretion is the rate of production – Steroid hormones require a stimulus for production, but once synthesized, their release occurs by passive diffusion.
Steroid hormones are not stored – in contrast to the amines (e.g. thyroid hormones –THYROXINE (T4), Triiodothryronine (T3)) , catecholamines (E.g. epinephrine, norepinephrine) and polypeptide hormones (e.g. pituitary ACTH, insulin,parathyroid hormone), steroid hormones are not packaged for storage for later use.
- Steroid hormone availability is thus dependent on their continual biosynthesis – to enable secretion to occur when needed for a physiological response or developmental change to occur.
What affects steroid production (Steroidogenesis)? Any chemical interference to steroidogenesis can cause adverse effects to the reproductive system. E.g., altering enzymatic activity or hormone production, altering precursor availability, interfering with control mechanisms, etc.
- Steroid hormone conversion enzymes
- Chronic stress, disease or nutritional status – secretion and activity of a particular hormone may be adjusted upward or downward in response to challenges such as chronic stress, disease, or a poor diet (excess sugar / refined starches, trans-fatty acids, lack of needed nutrients such as Omega-3 fats, a full range of essential amino acids, vitamins, minerals, etc.).
- Cholesterol availability – a constant supply of cholesterol is necessary for enzyme-catalysed steroid hormone production since they are manufactured on an “as needed”basis – unlike protein/polypeptide hormones, steroid hormones are not stored in large amounts – one of several reasons for not being too eager to lower your cholesterol levels. Cholesterol – Our Hero!
- StAR (Steroid Acute Regulator) protein. Transports cholesterol from the outer to the inner mitochondrial membrane – a rate-limiting step of steroidogenesis -cholesterol is carried between the membranes by the StAR protein and steroidogenesis is controlled through regulation of its production.
- StAR (Steroid Acute Regulator) Gene
- ▲ SF-1 (Steroidogenic Factor-1) regulates the basal and hormone-stimulated expression of the StAR gene, which codes the StAR protein that transfers cholesterol into mitochondria.
- ▲ Also upregulating the StAR gene and thus enhancing steroid production are: GROWTH HORMONE, ESTRADIOL, calcium, and IGF-1
- StAR pathway directly affected by: barbiturates, Lindane, DBA (byproduct of water disinfection using chlorine), Dimethoate, Diethylumbelliferyl phosphate, DMSO.
- StAR (Steroid Acute Regulator) Gene
- Hydrogen Peroxide (H2O2). Blocks conversion of: PREGNENOLONE ➔ PROGESTERONE;
- Dioxins (in fatty meats, dairy) ➔ low TESTOSTERONE, and limited prostate growth;
- Aminoglutethimide ➔ low TESTOSTERONE;
- Lead, cadmium ➔ low TESTOSTERONE;
- Cyclic-AMP Second Messenger System. C-AMP pathway directly affected by:
- Biphenol-A (BPA);
- Nicotine;
- Nitric oxide (NO);
- Glucocorticoids;
- Drugs – Lindane; Indmethacin. Chloroquine. EDS. DBA, Cotinine, Tylosin, Gossypol;
- Nitric oxide (NO). Blocks steroid production;

Most circulating steroid hormones are bound to steroid hormone binding carrier proteins
To make them water-soluble in the blood, most steroid hormones are bound to carrier proteins, and only a small fraction circulates “free”or unbound – A rapid equilibrium exists between protein-bound and unbound steroid in extracellular fluid.
- Protein-bound (enveloped by specific globulins or albumin) – when steroid hormones circulate through the liver, they eventually become protein-bound a process that not only impedes their bio-availability but also makes them water soluble, thus facilitating their excretion in urine.
NOTE: Measuring the concentration of these NON-bio-available forms in urine or serum is irrelevant since it provides no clue as to the concentration of the more clinically significant “free”(bio-available) hormone in the blood stream.
- Albumin – binds many steroids fairly loosely;
- Many steroid hormones have specific binding globulins:
- Sex Hormone Binding Globulin (SHBG) for estrogen and TESTOSTERONE;
- Corticosteroid Binding Globulin (CBG) for PROGESTERONE , CORTISOL (and other corticosteroids);
Unbound / “Free”- these are presumed to be the biologically active fraction available for entering targets cell through their lipid bilayer. Steroid hormones carry their message to cells by leaving the blood flow at capillaries to enter cells where they bond with specific hormone receptors.
Saliva testing is far superior to blood or urine testing in measuring “free”bio-available hormone levels – when circulating through saliva glands, the “free” non-protein-bound steroid hormone (whether in RBCs or serum) diffuses easily from blood capillaries into the saliva gland and then into the mucins of saliva. Protein-bound, non-bio-available hormones do not pass into or through the saliva gland

Steroid hormones act on target cells via receptors located in the cell interior
The primary function of steroid hormones is to affect an alteration in the rate of transcription of specific genes in target cells, by either increasing or decreasing their expression – The steroid hormone usually diffuses through the plasma membrane, through the cytoplasm and into the nucleus, where it binds to a receptor protein. In turn, this activates a portion of DNA that turns on specific genes.
There are several classes of steroid receptors – those for glucocorticoids, mineralocorticoids, sex steroids (which includes the androgens, estrogens and progestagens). Steroid receptors comprise a family of related proteins that also show homology to receptors for the thyroid hormones and vitamin D.
The receptor has regions or domains that carry out specific tasks:
- One for recognition and binding of the steroid
- Another for binding to a specific region on chromosomal DNA
- A third for helping regulate the transcriptional complex.
Steroid action is relatively slow in onset (hours) – but

Steroid hormone excretion
- Excretion in the urine, bile or feces. Steroid hormones that are excreted in the bile may be hydrolyzed in the G.I. tract and then reabsorbed back into the body through the portal system circulation;
- Steroids in the blood are eliminated by metabolism in the liver. There, reduced forms are produced and subsequently conjugated to glucuronic acid and sulfate. These metabolites are freely soluble in blood and are eliminated from the body via the kidneys and the GI tract.
- Small amounts of free steroid hormone are also directly excreted by the kidneys

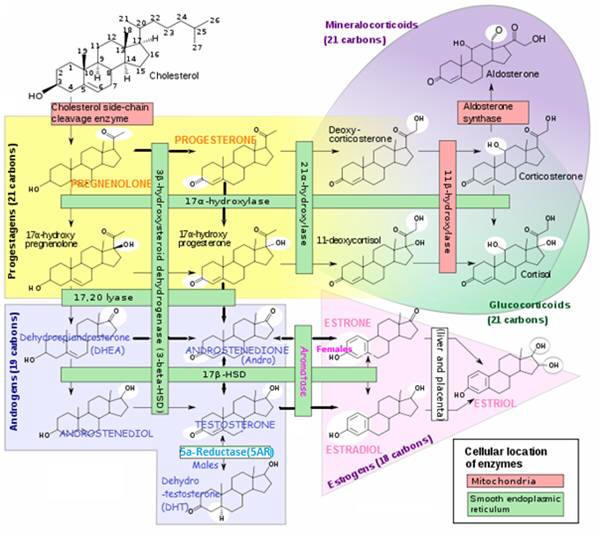
Why are steroid hormone conversion enzymes important ?
The conversion of one hormone to another is catalyzed by a certain enzyme. As depicted in the rectangular boxes in the following steroid conversion chart.
The level of an enzyme presence in a particular body tissue determines the extent to which the hormone conversion will take place. The amount of a certain hormone in particular areas is a critical factor affecting hormonally related health concerns.

At a Glance ChartEnzymes affecting Steroid Production/Activity and Conversion Pathways |
||||
| Enzyme | Converts |
Prod’n / Activity Conversion Pathway Increased by: |
Prod’n / Activity Conversion Pathway Decreased by |
Comments |
|
P450SCC Cholesterol side-chain cleavage enzyme; |
Cholesterol → PREGNENOLONE (in mitochondria); |
LH |
Estrogens; Lead; Vitamin A deficiency; Certain drugs |
Series of reactions occur at the inner membrane; |
|
P450 C17-20 (17α-hydroxylase) |
LH |
TESTOSTERONE (affects cAMP pathway) Ethanol; Nicotine; Certain drugs |
Catalyzes hydroxylation and cleavage (converts steroids from a 21-carbon to a 19-carbon molecule in Δ4 and Δ5- hydroxysteroid pathways); | |
|
17ß-HSD (17ß-Hydroxysteroid Dehydrogenase) |
DHEA → ANDROSTENEDIOL ANDROSTENEDIONE ⇔ TESTOSTERONE ESTRONE (E1) → ESTRADIOL (E2) |
Drugs: Cotinine, Danazol, Cyclosporin A, Lithium chloride. Lignans (E.g. in flaxseed) |
Lignans – Enterolactones (lignan precursors) found in E.g.flaxseed inhibit | |
|
3ß-HSD (3ß-Hydroxysteroid Dehydrogenase) |
PREGNENOLONE → PROGESTERONE DHEA → ANDROSTENEDIONE |
LH FSH via LH |
TESTOSTERONE; Lead; Daidzein /genistein /biochanin A (isoflavones); Aflatoxin |
In the male, FSH, stimulates release of a Sertoli cell factor that increases LH effect on 3ß-HSD activity; |
|
5AR (5α-Reductase) |
TESTOSTERONE → DIHYDRO-TESTOSTERONE (DHT) |
In testes, liver, brain, prostate, external genitalia, skin, hair follicles and sebaceous glands; DHT more potent than TESTOSTERONE; DHT involved in BPH,prostate cancer, hirsutism |
||
| Aromatase |
TESTOSTERONE → ESTRADIOL ANDROSTENEDIONE → ESTRONE (in females) |
Gonadotropins (E.g. FSH, LH, hCG); Age; Obesity; INSULIN; Alcohol. |
PROLACTIN; Smoking; Anti-mullerian hormone; Certain Drugs Finasteride; PROGESTERONE; Nettle root; Flavonoids |
In females, FSH increases aromatase activity, enhancing this conversion; |

Aromatase enzyme
Aromatase enzyme converts androgens to estrogens
Androgens ➔ Estrogens
aromatase enzyme
Aromatase is responsible for the production of 18-carbon estrogens from 19-carbon androgen steroids: TESTOSTERONE and ANDROSTENEDIONE – the gene CYP19 encodes the aromatase enzyme (a member of the Cytochrome P450 enzyme superfamily).
ANDROSTENEDIONE ➔ ESTRONE (in women)
(In the female, FSH increases aromatase activity, enhancing this conversion)
TESTOSTERONE ➔ ESTRADIOL
Aromatase production
Different body tissues produce estrogen via aromatase response to different activating promoters. Tissue-specific regulation of CYP19 (aromatase gene) expression is achieved through the use of distinct promoters controlled by hormones and other factors, including:
- Glucocorticoids (E.g. CORTISOL, the drug prednisone),
- Class 1 cytokines: E.g. IFN-γ, IL-2, IL-6, IL-11, and TNF-α(present in infection);
- Retinoids. E.g. vitamin A
- FSH
Aromatase Gene (CYP19) Expression Regulators | ||
| Location | Promoter | Promoter regulated by: |
| Ovaries | Cyclic AMP via the proximal promoter II | FSH |
| Placenta | distal promoter I.1 | Retinoids |
| Adipose tissue, skin fibroblasts, bone | distal promoter I.4 | Glucocorticoids and class 1 cytokines E.g. tumor-derived PGE2 is the major factor stimulating local aromatase expression in the breast fat of cancer patients. |
Aromatase can be found in several tissues
- Tissues having beneficial estrogen presence – includes gonads, brain, adipose tissue (E.g. found in the breast), placenta, blood vessels, skin, bone, and endometrium.
- Health conditions where inappropriately high levels of aromatase are present in tissues – endometriosis, uterine fibroids, breast cancer(enhanced aromatase activity found in breast fat)and endometrial cancer.
Aromatase ACTIVITY is inhibited by (examples):
- Nettle root
- PROGESTERONE
- Drugs: MEHP (monoethylhexylphthalate); fenarimol; substituted analogs of ANDROSTENEDIONE, e.g., C-10 substituted (19R-10 β-oxiranyl-), or C-4 substituted (OH-, formestone), or C-7 substituted (7 α-SC6H4-p-NH2-), or 2 β, 19 bridged (2 β, 19-methylene-), fadrazole, letrozole, and arimidex,
Aromatase ACTIVITY is ▲Enhanced by:
- Age.
- Obesity.
- INSULIN.
- Gonadotropin hormones (E.g. FSH, LH, hCG);
- Alcohol.
- Location in certain estrogen-dependent local tissue E.g. next to breast tissue
- Endometrial cancer, endometriosis, and uterine fibroids.
Aromatase ACTIVITY is ▼Reduced by:
- PROLACTIN (stimulates milk production for breast-feeding);
- Smoking.
- Anti-mullerian hormone (AMH). Produced by reproductive tissues. Inhibits the development of the Mullerian ducts in the male embryo, which develop into reproductive organs in females.
- Drugs (post-menopausal only) Letrozole (Femara®), anastrozole (Arimidex®), and exemestane (Aromasin®).
Aromatase conversion pathway directly affected by:
- Flavonoids and lignans (both polyphenols). Enterolactones are lignan precursors found in E.g.flaxseed;
- Drugs. Aminoglutethimide, MEHP, Fenarimol, Fadrazole, Letrozole, Anastrozole, Arimidex, Prochloraz, Enconazole/miconazole/ketoconazole, Imizolil, 4-hydroxyANDROSTENEDIONE, 10-propargylestr-4-ene-3,17-dione

5α-Reductase (5AR) enzyme
5α-Reductase converts:
TESTOSTERONE➔ DIHYDROTESTOSTERONE (DHT) (normally in males)
Mainly in peripheral tissue, but also in testes; DHT is significantly more potent than TESTOSTERONE, and thought to be involved in prostate enlargement, prostate cancer and PCOS.
There are two 5AR forms (isoenzymes)
- 5-alpha reductase 1 (SRD5A1) – used to create neurosteroids
- 5-alpha reductase 2 (SRD5A2) – converts TESTOSTERONE ➔ DHT
5AR Inhibited by:
- PROGESTERONE
- Drugs (E.g. Finasteride, Dutasteride) – used against BPH, prostate cancer and male pattern baldness
- Saw Palmetto (Serenoa repens)
- Willow herb – contains oenothein B, with 5AR inhibitor activity
- Astaxanthin – this carotenoid has demonstrated some 5AR inhibitor activity
- Black cohosh (Cimicifuga racemosa) Maturitas. 2006.Department of Clinical and Experimental Endocrinology, University of Goettingen, Robert-Koch-Strasse, Gottingen, Germany
- 5AR inhibitor (5ARI) drugs (E.g. Finasteride, Dutasteride) have been associated with adverse outcomes, which side-effects may also be experienced with any natural 5AR inhibitor:
- Adverse sexual outcomes – such as lack of libido, erectile dysfunction (ED), and ejaculatory dysfunction (EjD). Since Nitric Oxide (NO) is required to maintain an erection, a suggested theory is that lack of DHT inhibits nitric oxide synthase (NOS) activity.
- Anxiety / Depression – common side effects of 5ARI s believed to be caused, in part, by the prevention of the endogenous production of the neurosteroid allopregnanolone from PROGESTERONE
- Cancer
- Finasteride – inhibits the function of only type 2 form of 5AR (SRD5A2); however, it was found in a study of 18,000 healthy men, that although the finasteride group had a lower incidence of cancer (18.4% vs. 24.4%) there were more cases of aggressive cancer with finasteride than with placebo group (37% vs. 22.2%, P<0.001). Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215-224 ;
- Dutasteride – inhibits both forms of 5AR (SRD5A2 and SRD5A2);

P450SCC enzyme
At the inner membrane, a series of enzymatic reactions occurs, Enzyme P450SCC converts:
Cholesterol ➔ PREGNENOLONE (in mitochondria)
Production / Activity of P450SCC regulated by:
- LH
P450SCC inhibitors include:
- Estrogens
- Drugs: Aminoglutethimide, 3-methoxybenzidine, cyanoketone, azastine, and danazol.
- P450SCC pathway directly affected by:
- Lead;
- Vitamin A deficiency;
- Drugs: Ketoconazole, Mibolerone, Aminoglutethimide, Taxol, Cis-platinum,

<span class="threeB-HSD"3ß-HSD (3ß-Hydroxysteroid Dehydrogenase) enzyme
3ß-HSD converts:
PREGNENOLONE ➔ PROGESTERONE
DHEA ➔ ANDROSTENEDIONE;
3ß-HSD Production/Activity stimulated by:
- LH
- FSH (in the male) – stimulates release of a Sertoli cell factor that increases LH effect on 3ß-HSDactivity;
3ß-HSD production / activity suppressed by:
- TESTOSTERONE (through inhibitory effects on cAMP-mediated 3ß-HSD mRNA)
3β-HSD pathway by:
- Lead;
- Daidzein /genistein /biochanin A (isoflavones)
- Drugs: Lithium chloride, Mibolerone, Danazol (ethinylTESTOSTERONE), Cyproterone acetate, Ethionine, Cyanoketone, Mitomycin C, Aflatoxin

P450 C17-20 (17α-hydroxylase) enzyme
17α-hydroxylase (P450c 17-20) catalyzes hydroxylation and cleavage of steroids (converts steroids from a 21-carbon to a 19-carbon molecule in Δ4 and Δ5- hydroxysteroid pathways);
Production / Activity Regulated by:
- LH
P450c17 activity suppressed by:
- TESTOSTERONE (via effects on second messenger cAMP pathway)
17α-hydroxylase /C17-20 lyase pathway directly affected by:
- Nicotine;
- Ethanol (17α-hydroxylase)
- Drugs: Bromocriptine, Mibolerone, Danazol, Cyproterone acetate, Cyclosporin A, Flutamide.
Δ5 – hydroxy-steroid pathway | PREGNENOLONE | → P450 C17 hydroxylation | 17α-Hydroxy- PREGNENOLONE | → P450 C17 Lyase activity cleavage | |
Δ4 – hydroxy-steroid pathway | PROGESTERONE | → P450 C17 hydroxylation | 17α-Hydroxy-PROGESTERONE | → P450 C20 Lyase * | ANDRO-STENEDIONE |
* In rats, P450 C17 catalyses: 17α-Hydroxyprogesterone ➔ ANDROSTENEDIONE (but not in humans, which could explain differences between rat and human studies);

Enzyme 17ß-HSD (17ß-Hydroxysteroid Dehydrogenase) enzyme
17ß-HSD converts:
ANDROSTENEDIONE <=> TESTOSTERONE
ESTRONE (E1) <=> ESTRADIOL (E2)
ESTRADIOL (E2) synthesis occurs principally through oxidation of ESTRONE (E1) – and is a reversible reaction;
The ESTRADIOL (E2) / ESTRONE (E1) ratio before menopause is > 1 – after menopause ESTRONE 1 is the predominant estrogen.
Both ESTRADIOL (E2) and ESTRONE (E1) can be metabolized to the biologically weaker ESTRIOL (E3), and to a number of other metabolites – via A-ring (Anti-carcinogenic) metabolic pathways, E.g, 2-HYDROXYESTRONE and its methylated form 2-METHOXYESTRONE or D-ring (potentially carcinogenic) metabolism E.g, 16α- HYDROXYESTRONE.
Tissue enzymes (E.g sulfatases and 17ß-HSDs) originating in bowel bacterial flora and the intestinal mucosa, activate strogens locally to ESTRADIOL (E2) and ESTRONE (E1). Stanczyk F. Estrogens used for replacement therapy in postmenopausal women. Gynecol Endocrinol. 2001;15(suppl 4):17-25.
17ß-HSD pathway directly affected by:
- Lignans
- Enterolactones (lignan precursors) found in E.g.flaxseed inhibit
- Drugs: Cotinine, Danazol, Cyclosporin A, Lithium chloride.











![<span class="ACETYLCHOLINE">Acetylcholine (Ach)</span><span class="hdg-smaller"> – Movement, memory, learning neurotransmitter</span> – [Cloned #60543]](https://healthhappening.com/wp-content/uploads/2025/12/Ach.jpg)