Typical processing of most oils damages / alters their fatty acids making them toxic

Oxygen will damage / alter unsaturated fatty acids in seed, nut and bean oils (Exacerbated by heat and especially light)
Light increases the damage caused by oxygen by 1000-fold
Polyunsaturated fats (mainly EFAs) should NEVER be heated above 320°F
Most commercial oils containing predominately polyunsaturated fats are processed by being solvent-extracted, refined, bleached and deodorized, and some are additionally hydrogenated or partially hydrogenated (see below) to:
- Prolong shelf life (by preventing or retarding rancidty)
- Produce the right “taste-feel”
- Produce the right consistency for spreading (as in margarine, shortening) or for use by food processors.
Consumed toxic fats are now well-known to wreak havoc in the body by causing cellular dysfunction and are very much involved in today’s familiar health problems.
They can damage arteries and other body parts if not neutralized by free-radical protecting, antioxidant supplies, such as the fat-soluble vitamins A, D, E and K, which if used to depletion, are then no longer available for their usual protective functions in the body.
Oxygen / peroxidation. Oil is damaged when oxygen combines with the weak carbon bonds of its unsaturated fatty acids, causing them to break apart into fragments, called lipid PEROXIDES. These create free radicals, whose unpaired electrons, start a chain reaction which can propagate hundreds of thousands more free radicals and possible broken double carbon bonds, ultimately causing the oil to become rancid.
Light. Light can increase peroxidation rate by up to 1000 times.
Heat. Trans fatty acids (tFAs) and other altered, unnatural fatty acids start to form when heated over 320°F. At 392°F they form substantially, and at 428°F they form exponentially. Unsaturated fatty acids become mutagenic (i.e. can damage our genes) when heated above 320°F. Usual grocery-store, solvent-extracted polyunsaturated oils have typically experienced a temperature of 464 – 482° F in the steam distillation /deodorization stage of processing, which forms tFAs from unsaturated oils at about 1% or less per hour. [Reference]
The high heat used (464 – 482° F) during the deodorization step during oil processing not only removes the health-precious essential fats, and other healthy components, it also introduces toxic, unnatural trans fats (tFAs) and lipid peroxides into oils containing unsaturated fats. Corn, soybean, sunflower, cottonseed and other refined polyunsaturated -rich oils are sold to the consumer in the grocery store, in processed foods, and served at restaurants, often after further assault in a deep fryer! (Typical frying temp. is 350° – 375°)
Hydrogenated or partially hydrogenated fat
- What's that mean?
Hydrogenated fat
Hydrogenation produces artificially saturated fats and partially hydrogenated fats (E.g. Trans fats)
Hydrogenation (saturation) of unsaturated oils retards or eliminates the potential for these oils to go rancid for several years, makes a product “stiffer”, and also provides spreadability, texture and “mouth feel”. Unsaturated fats exposed to light, heat or air otherwise tend to oxidize and go rancid easily.
Hydrogenation is a commercial chemical process. Patented in Germany in 1902, hydrogenation decreases the number of double bonds in unsaturated fatty acids of predominately unsaturated oils, such as soybean, corn or sunflower oil, these fully refined oils are then heated to high temperatures (248°- 410°F) with metal catalysts, usually nickel or 50/50 nickel/aluminum, in the presence of pressurized hydrogen gas. (The aluminum is a concern since its presence in the body is associated with Alzheimer’s, osteoporosis + more). This enables the hydrogen to be added into (i.e. saturate) the double bonds of the unsaturated fatty acids, and since they then contain no double bonds, makes them relatively chemically inert.
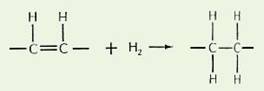
Hydrogenated fats also contain partially hydrogenated fats, such as trans fats. Since the hydrogenation process never achieves 100% efficiency, leaving some partially hydrogenated (altered / toxic) fats.
Foods containing hydrogenated fats – include margarine, shortening (E.g. Crisco®)
Partially hydrogenated fat
Partial hydrogenation produces altered/toxic fatty acids (E.g. Trans Fats)
Fully saturated fats are usually too waxy and solid to add to food processing steps. So manufacturers typically require partially hydrogenated oils, for which the hydrogenation process is stopped when the oil has the proper consistency for its application. This process is used to make products like chocolate, “hard enough to melt in your mouth, but not in your hand!” Unfortunately, the partial hydrogenation process also results in the presence of dozens of altered, intermediate substances including trans fats, conjugated fatty acids, double bond isomers (double bonds relocated to new, unnatural positions) and fatty acid fragments.
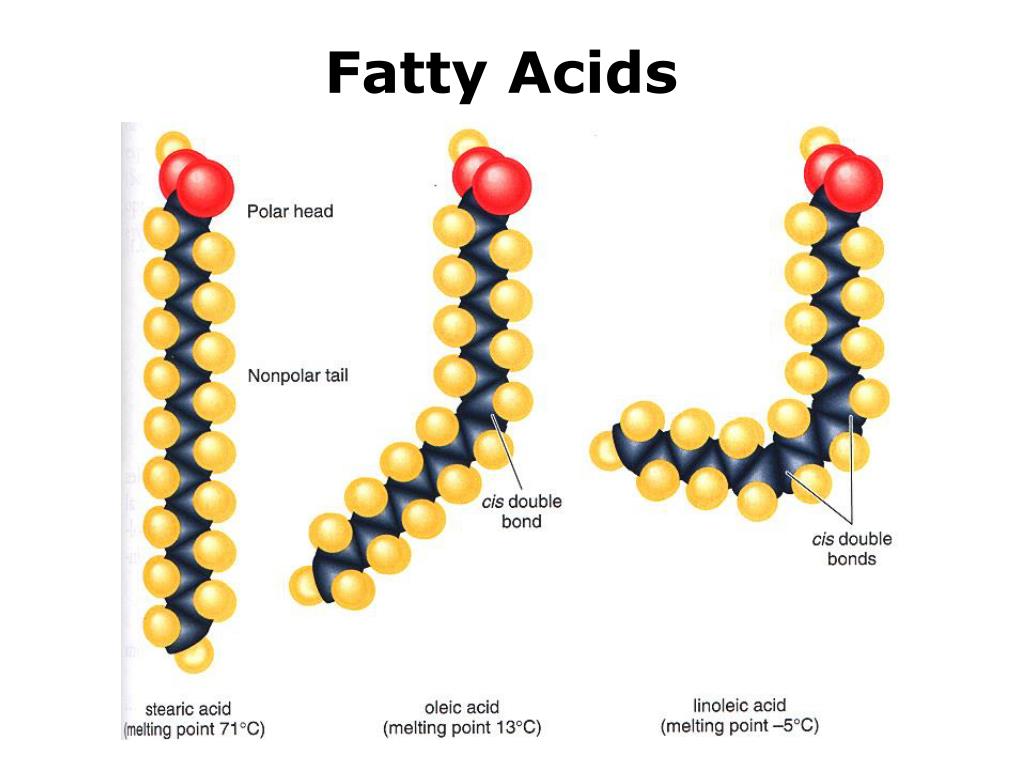
A NATURAL, unsaturated fatty acid is in what is called the CIS configuration. With the hydrogen atoms at a double bond on the same side of the molecule. This lack of symmetry forces a kink or bend in the carbon chain
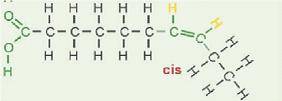
Trans fatty acids
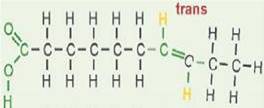
A TRANS configuration fatty acid is produced by heat-processing during hydrogenation, frying in unsaturated oils, and the high temperatures in the oil refining process. The TRANS configuration arises as a hydrogen atom TRANSfers to the other side of the fatty acid molecule. The TRANS configuration fatty acid has only a slight kink in its carbon chain. tFAs begin to form above 320°F, and form in substantial quantities above 392 °F.
High levels of 30-50% trans fatty acids (tFAs) are commonly found in these oils:
• Highly processed oils. E.g. margarine, shortening
• Oils used for repeated frying. In restaurants oils (called “liquid shortening”)
• Partially-hydrogenated vegetable oils”. Found in ready-made french fries and many packaged goods, such as commercially processed foods/ Foods with a long shelf-life – such as cookies, cakes, crackers, bread, candies, most peanut butter, pancake mixes, instant soups, chocolate, some salad dressings, junk foods, chips, croutons, granola bars.
Don't believe the “0 TRANS FAT” Label!
Some trans fats occur in nature and are health beneficial.
(e.g. conjugated linoleic acid (CLA) in the butter of grass-fed cows)
However, they are very different to those introduced during commercial oil processing. Unsaturated fats in plants eaten by ruminants undergo biohydrogenation by bacteria, catalyzed by bacterial enzymes at normal body temperature and pressure. In contrast, the industrial hydrogenation process is initiated by metal catalysts under very high temp and pressure producing trans fats not encountered in nature, whose isomers have vastly different distribution, causing them to have very different effects.