TESTOSTERONE - A predominately male hormone


Summary
TESTOSTERONE is a sex steroid hormone with tissue-building and androgenic (masculizing) effects:
- Responsible for male characteristics;
- Plays a large role in male sexual development
- Influences libido
- Regulates basic metabolism
- Stimulates red blood cell production
- Hinders excessive production of free radicals
- Facilitates protein synthesis / Builds body tissues
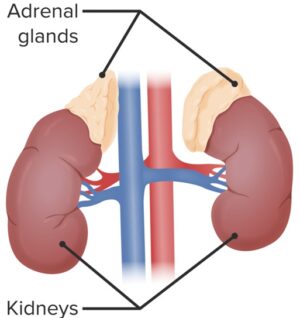
TESTOSTERONE is produced in testes, ovaries, and adrenal cortex
TESTOSTERONE directly activates androgen receptors (ARs). Can affect changes in muscle and brain.
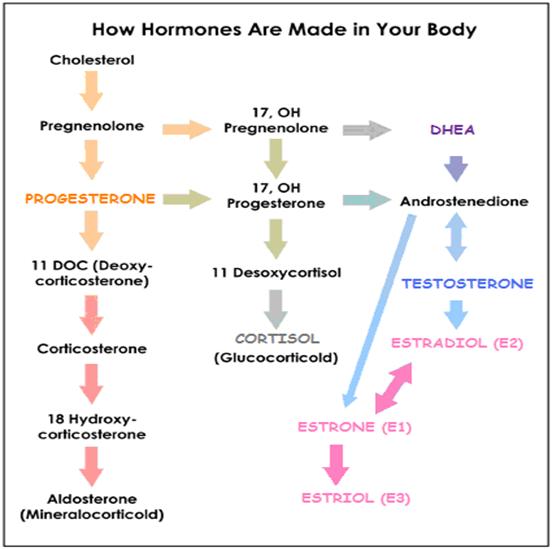
TESTOSTERONE converts to:

TESTOSTERONE functions
Responsible for male characteristics.
In male puberty – aids:
- Growth of penis, testes, facial and pubic hair
- Deepening of the voice
- Increase in muscle mass /strength -by increasing muscle tissue nitrogen retention rate, TESTOSTERONE promotes muscle growth and also prevents muscle breakdown
- Increase in height;
In adult male – maintains:
- Sex drive
- Sperm production – Men start producing sperm at puberty and continue to produce them for the rest of their life
- Male hair patterns
- Muscle mass
- Bone density
Lowers blood sugar
TESTOSTERONE functions in women
- Contributes to sex drive;
- Helps maintain muscle mass and build bone.

TESTOSTERONE production
TESTOSTERONE production / secretion in Men
Source of TESTOSTERONE production / secretion :
- >95% of circulating TESTOSTERONE secretedby the Lehdig cells of the testes (6-8mg/day)
- A small amount produced by adrenal cortex
TESTOSTERONE production decreases steadily with age (called Andropause) – with a concurrent increase in estrogen levels;
- TESTOSTERONE and estrogen are antagonists – and one particular estrogen, ESTRADIOL, is known to turn on the BCL2 cancer gene, increasing the risk of prostate cancer, when there is an inadequate amount of PROGESTERONE to counteract estrogen’s effect by stimulating the P53 cancer protection gene.
- TESTOSTERONE production decreases 1-2% /yr from age 30 to 70in the U.S. Research by National Institutes of Health
Testes secrete higher levels of TESTOSTERONE in the morning and lower levels in the evening – interestingly this seems to be an intrinsic circadian rhythm, since it is not accompanied by changes in Luteinizing Hormone (LH).
TESTOSTERONE production / secretion control
- GnRH from hypothalamus stimulates pituitary secretion of Luteinizing Hormone (LH) – which stimulates secretion of TESTOSTERONE from testes.
Hypothalamus → GnRH → Pituitary → LH → Testes → TESTOSTERONE
- By negative feedback, elevated levels of TESTOSTERONE suppress the release of hypothalamic GnRH – ESTRADIOL also suppresses GnRH.
- TESTOSTERONE production is also inhibited by: the inhibition of LH output, by circulating non-SHBG-bound TESTOSTERONE, and especially by DHT (to provide negative feedback control of its own production, which is stimulated by LH); although, even in men, the major hormone involved in LH feedback is ESTRADIOL, not TESTOSTERONE; (SHBG is Sex Hormone Binding Globulin)
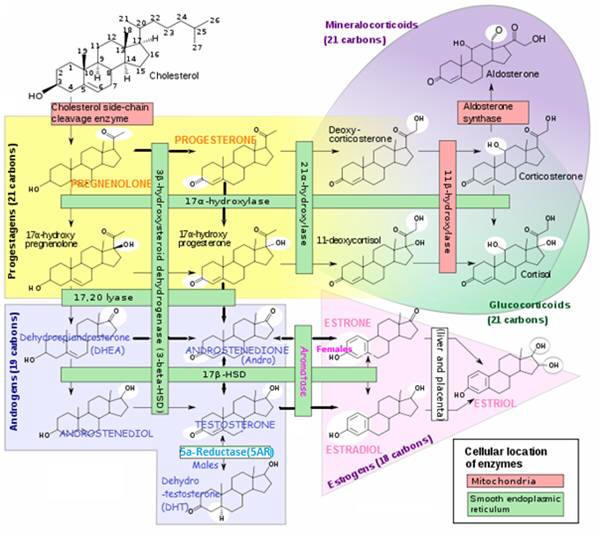
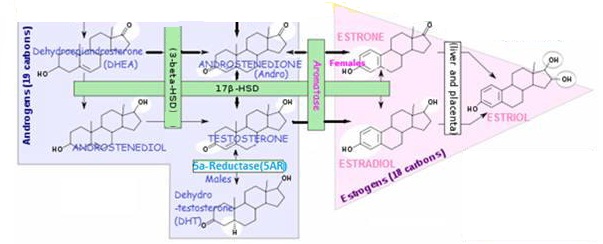
TESTOSTERONE is synthesized via two pathways:
- Δ4 pathway – From ANDROSTENEDIONE (via 17-βhydroxysteroid dehydrogenase enzyme) (preferred pathway)
- or Δ5 pathway – From ANDROSTENEDIOL (itself synthesized from DHEA)
TESTOSTERONE production / secretion in Women
Source of TESTOSTERONE production is roughly:
- 50% from peripheral conversion
- 25% by the ovaries (thecal cells)
- 25% by adrenal cortex
- The placenta also produces TESTOSTERONE during pregnancy
- An overall total of ~ 0.3 mg TESTOSTERONE / day, ~1/7th – 1/10th the amount produced by men.
- The ovaries of a normal ovulatory woman secrete ~0.05-0.1 mg TESTOSTERONE / day;
During reproductive years most TESTOSTERONE produced by ovaries is converted to ESTRADIOL
Post-menopausal TESTOSTERONE production decreases gradually – to only 1/3 of premenopausal amounts (estrogen decreases more dramatically);
Removing ovaries causes TESTOSTERONE production to fall by 25% – i.e. leaving only adrenal and peripheral production.
Estrogen replacement therapy reduces TESTOSTERONE production.
TESTOSTERONE production control
- GnRH from hypothalamus stimulates pituitary secretion of Luteinizing Hormone (LH) – which stimulates secretion of TESTOSTERONE from ovaries.
Hypothalamus → GnRH → Pituitary → LH → Ovaries → TESTOSTERONE
- By negative feedback, elevated levels of TESTOSTERONE suppress the release of hypothalamic GnRH – ESTRADIOL also suppresses GnRH;
TESTOSTERONE production is also inhibited by: the inhibition of LH output,by circulating non-SHBG-bound TESTOSTERONE, and especially by DHT (to provide negative feedback control of its own production, which is stimulated by LH); although, even in men, the major hormone involved in LH feedback is ESTRADIOL, not TESTOSTERONE; (SHBG is Sex Hormone Binding Globulin)
TESTOSTERONE is synthesized via two pathways:
- Δ4 pathway – From ANDROSTENEDIONE (via 17-βhydroxysteroid dehydrogenase enzyme) (preferred pathway)
- Δ5 pathway – From ANDROSTENEDIOL (itself synthesized from DHEA)

Health effects / symptoms of high TESTOSTERONE
Health Effects of high TESTOSTERONE (hyperandrogenism) mainly attributed to TESTOSTERONE’s conversion to the more potent DHT by 5AR enzyme:
Health problems associated with High or Low DHT levels
Health effects of high TESTOSTERONE (hyperandrogenism) in men
Possible EFFECTS /SYMPTOMS of high TESTOSTERONE in MEN
- Aggressive behavior
- Fuels proliferation of prostate cancer cells
- Causes red blood cells to clump together and thicken blood – increasing risk for stroke
Health effects of high TESTOSTERONE (hyperandrogenism) in women
Health Problems Associated with High or Low DHT levels in Women
- Changes a woman’s physique to look more like a man – symptoms include acne, fat loss (and a redistribution of fat storage throughout the body). increased facial hair growth, increased perspiration, head hair loss, and decreased HDL;
- Altered libido
- Disrupted menstrual cycles
- Enlarged clitoris

Effects / Symptoms of low TESTOSTERONE (Androgen Deficiency)
Effects / Symptoms of low TESTOSTERONE (Androgen deficiency) in men
- Decreased sex drive (libido)
- Erectile dysfunction / Inadequate erections – ~65% of men ages 70-79 are impotent.
- Increased breast size and tenderness – due to low TESTOSTERONE to estrogen ratio
- Afternoon fatigue
- Symptoms similar to menopause in women (e.g., hot flashes, increased irritability, inability to concentrate, depression)
- Abdominal fat → aromatase production → estrogen ▲ → even lower TESTOSTERONE ▼ (by aromatization of TESTOSTERONE → ESTRADIOL)
Effects / Symptoms of Severe Androgen deficiency:
- Loss of armpit and pubic hair
- Loss of muscle mass / Replaced with fat (since TESTOSTERONE helps with protein synthesis for muscle-building); loss of physical strength endurance.
- Fragile bones
- Small, soft testes
- Low sperm count – male infertility
Lab Results with low TESTOSTERONE
- Decreased bone density by dexa scan
- Loss in height of more than 1 inch
- High blood pressure and heart enlargement with associated chest pain
- Increase in abdominal girth with 40″as maximum for men and waist size of in excess of 34 inches in women
- Low Free TESTOSTERONE, occasionally low total TESTOSTERONE, and low normal bioavailable TESTOSTERONE
- Loss of penile reflexes and decreased penis sensitivity
- Lowered sperm count and fertility
- INSULIN resistance
- High blood glucose
- Below normal HDL
- Low Sex Hormone Binding Globulin (SHBG)
Effects / Symptoms / Causes of low TESTOSTERONE (Androgen deficiency) in women
Effects / Symptoms of low TESTOSTERONE in women:
- Menopausal women with decreased libido.
- Menopausal women with advanced osteoporosis.
- Depression, memory loss
- Bone loss
- Vaginal dryness
- Incontinence
Causes of low TESTOSTERONE in women:
- Women who have had their ovaries removed
- Women who have lost pituitary function (as a result of surgery or certain medical problems) – pituitary normally releases luteinizing hormone (LH) to stimulate TESTOSTERONE production.
TESTOSTERONE HRT for WomenTESTOSTERONE therapy for menopause – TESTOSTERONE as a component of hormone replacement therapy (HRT) for postmenopausal women is used mostly for women who complain of loss of sexual interest and libido. Davis SR, McCloud PI, Strauss BJG & Burger HG, TESTOSTERONE enhances ESTRADIOL’s effects on post- menopausal bone density and sexuality. 1995, Maturitas 21 227-236. Postmenopausal TESTOSTERONE replacement is demonstrating effectiveness in prevention / treatment of osteoporosis Raisz LG, Wiita B & Artis A Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. 1995 Journal of Clinical Endocrinology and Metabolism 81 37-43 TESTOSTERONE for endometriosis – TESTOSTERONE added to cultured ovarian granulosa cells from reproductive age women with endometriosis reduced aromatase activity and reduced basal production of ESTRADIOL in vitro. Gynecological Endocrinology, 2006Gynecological Endocrinology, 2006 |

TESTOSTERONE metabolism
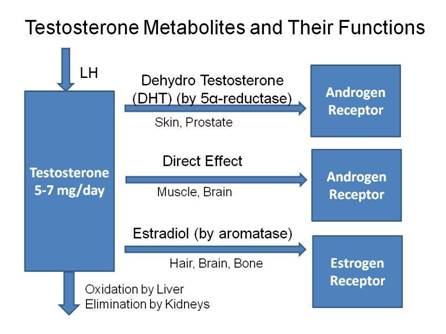
Androgen responses when binding to Androgen Receptor (AR) IN TISSUES
Circulating TESTOSTERONE provides the precursor for conversion to DHT and ESTRADIOL. Local DHT levels may be up to 10 times the TESTOSTERONE levels in tissues with a high 5α-Reductase (5AR) enzyme presence, such as the prostate. Hay ID, Wass JA (2009)
In tissues containing 5α-Reductase (5AR) enzyme (highly expressed in the prostate gland) – ~7% of TESTOSTERONE is converted to the more potent androgen DHT, which activates the AR leading to a 2-3 times stronger androgenic response than from TESTOSTERONE;
- Androgen target tissues include skin and prostate
- A major metabolite of DHT is 3B-androstenediol via enzyme 3B-HSD in various tissues, such as muscle, adipose and liver, which is conjugated to glucuronic acid in the liver for renal excretion.
- Unlike TESTOSTERONE, DHT is inactivated by the enzyme 3α-HSD into a very weak androgen 3α-ANDROSTANADIOL
In tissues NOT containing 5AR – TESTOSTERONE initiates a direct androgenic response when binding to the androgen receptor (AR) – by inducing expression of androgen-dependent genes;
In tissues containing aromatase – TESTOSTERONE converts to ESTRADIOL (and ANDROSTENEDIONE converts to ESTRONE in females) in estrogen responsive tissues (i.e. those containing Estrogen Receptors (ER’s)), including skin and liver. This may:
- Exert an effect in situ if tissue is estrogen – responsive or
- Enter plasma for distribution to peripheral target tissues – mainly adipose tissue (in men or women)
TESTOSTERONE Metabolites:
- Dihydrotestosterone (DHT) – in testes, liver, brain, prostate, external genitalia, skin, hair follicles and sebaceous glands (via 5AR enzyme);
- ESTRADIOL – in testes (~ 1/3 of male circulating ESTRADIOL), ovaries, liver, fat, muscle, brain (via aromatase enzyme; male levels of estrogen tend to increase with age (at the expense of TESTOSTERONE) due to increased aromatase activity;
- ANDROSTENEDIONE – a weak androgen, ~1/7 potency of TESTOSTERONE
- ANDROSTENEDIOL
- Glucuronide – in liver for renal elimination
- Etiocholonolone

TESTOSTERONE in the blood
TESTOSTERONE (like all sex steroids) is transported in blood bound primarily to either albumin or Sex Hormone-Binding Globulin (SHBG)
In Men
- NOT available for tissue uptake – SHBG-bound (44-65%) -The plasma level of SHBG (Sex Hormone Binding Globulin) approximately equals TESTOSTERONE levels (~25nm, ~22nM resp.);
- Available for tissue uptake – Non-SHBG-bound (33-50%) most reversibly bound to albumin; ~4% to cortisol-binding globulin;~2% free / unbound;
In Women
- NOT available for tissue uptake – SHBG-bound (66-78%) The plasma level of SHBG (Sex Hormone Binding Globulin) approximately equals TESTOSTERONE levels (~25nm, ~22nM resp.);
- Available for tissue uptake – Non-SHBG-bound (20-30%) – most reversibly bound to albumin; ~4% to cortisol-binding globulin;~2% free / unbound;
An increase in TESTOSTERONE or decrease in SHBG level results in more TESTOSTERONE available for tissue uptake – in contrast, hypogonadal men with diminished gonadal function /low TESTOSTERONE production and those who are hyperthyroid have elevated SHBG.
What affects blood TESTOSTERONE levels?
High TESTOSTERONE production caused by:
- Low estrogen
- PCOS (polycystic ovary syndrome)
- Ovarian cancer
- Using anabolic steroids – for sports/bodybuilding
- Quercetin (e.g. in red wine) inhibits aromatase (enzyme for estrogen production) thus elevating TESTOSTERONE
- Antioxidants (vitamin A, and E, zinc, and selenium) – all support TESTOSTERONE production
Low TESTOSTERONE production caused by:
- High level estrogen
- Smoking
- Aging – lehdig cells in testes become less sensitive to luteinizing hormone (LH);
- Chronic illness
- Delayed puberty
- Hypopituitarism
- Prolactinoma
- Testicular failure in men
LOW TESTOSTERONE LEVELS can also be caused by:
- Excessive alcohol consumption – increases estrogen levels in men
- Obesity – enzyme aromatase in fat tissue converts TESTOSTERONE to ESTRADIOL thus lowering TESTOSTERONE;
- Chronic high stress – causes TESTOSTERONE to be converted to DHEA
- Increased SHBG – Steroid Hormone Binding Globulin binds TESTOSTERONE in blood, thus lowering available free TESTOSTERONE;

References
Hay ID, Wass JA (2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 37
Medical Biochemistry by N. V. Bhagavan
Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (5th Edition) by Carl A. Burtis, Edward R. Ashwood, David E. Brun. Elsevier Health Sciences, Oct 14, 2012
E Simpson, G Rubin, C Clyne, K Robertson, L O’Donnell, S Davis and M Jones, Local estrogen biosynthesis in males and females, Endocrine-Related Cancer (1999) 6 131-137